Lepidoptera: Lycaenidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Callophrys Gryneus (Juniper Hairstreak)
Maine 2015 Wildlife Action Plan Revision Report Date: January 13, 2016 Callophrys gryneus (Juniper Hairstreak) Priority 2 Species of Greatest Conservation Need (SGCN) Class: Insecta (Insects) Order: Lepidoptera (Butterflies, Skippers, And Moths) Family: Lycaenidae (Gossamer-winged Butterflies) General comments: Only 2-3 populations – previously historic; specialized host plant; declining and vulnerable habitat. Species Conservation Range Maps for Juniper Hairstreak: Town Map: Callophrys gryneus_Towns.pdf Subwatershed Map: Callophrys gryneus_HUC12.pdf SGCN Priority Ranking - Designation Criteria: Risk of Extirpation: Maine Status: Endangered State Special Concern or NMFS Species of Concern: NA Recent Significant Declines: NA Regional Endemic: NA High Regional Conservation Priority: NA High Climate Change Vulnerability: NA Understudied rare taxa: Recently documented or poorly surveyed rare species for which risk of extirpation is potentially high (e.g. few known occurrences) but insufficient data exist to conclusively assess distribution and status. *criteria only qualifies for Priority 3 level SGCN* Notes: Historical: NA Culturally Significant: NA Habitats Assigned to Juniper Hairstreak: Formation Name Cliff & Rock Macrogroup Name Cliff and Talus Habitat System Name: North-Central Appalachian Acidic Cliff and Talus **Primary Habitat** Notes: where host plant (red cedar) present Habitat System Name: North-Central Appalachian Circumneutral Cliff and Talus Notes: where host plant (red cedar) present Formation Name Grassland & Shrubland Macrogroup -

Acta Zool. Hung. 53 (Suppl
Acta Zoologica Academiae Scientiarum Hungaricae 53 (Suppl. 1), pp. 211–224, 2007 THE DESCRIPTION OF THERITAS GOZMANYI FROM THE ANDES AND ITS SPECTROSCOPIC CHARACTERIZATION WITH SOME NOTES ON THE GENUS (LEPIDOPTERA: LYCAENIDAE: EUMAEINI) BÁLINT, ZS.1, WOJTUSIAK, J.2, KERTÉSZ, K.3 and BIRÓ, L. P.3 1Department of Zoology, Hungarian Natural History Museum H-1088 Budapest, Baross u. 13, Hungary; E-mail: [email protected] 2Zoological Museum, Jagiellonian University, 30–060 Kraków, Ingardena 6, Poland 3Department of Nanotechnology, Research Institute for Technical Physics and Material Science H-1525 Budapest, P.O. Box 49, Hungary A key for separating sister genera Arcas SWAINSON, 1832 and Theritas HÜBNER, 1818, plus eight nominal species placed in Theritas is given. Three species groups within the latter genus are distinguished. A new species, Theritas gozmanyi BÁLINT et WOJTUSIAK, sp. n. is described form Ecuador. The presence of a discal scent pad on the fore wing dorsal surface and spectral characteristics of the light reflected from the central part of the discal cell were used as charac- ters for discrimination of the new species. Key words: androconial clusters, spectroscopy, structural colours, Theritas species-groups INTRODUCTION The generic name Theritas was established by monotypy for the new species Theritas mavors by HÜBNER (1818). The genus-group name was not in general use until the revision of D’ABRERA (1995), who placed 23 species-group taxa in Theritas on the basis of the character “the pennent-like tail which projects out- wards, and an approximate right angle from, and as a part of, a squared-off projec- tion of the tapered h. -

Pine Island Ridge Management Plan
Pine Island Ridge Conservation Management Plan Broward County Parks and Recreation May 2020 Update of 1999 Management Plan Table of Contents A. General Information ..............................................................................................................3 B. Natural and Cultural Resources ...........................................................................................8 C. Use of the Property ..............................................................................................................13 D. Management Activities ........................................................................................................18 E. Works Cited ..........................................................................................................................29 List of Tables Table 1. Management Goals…………………………………………………………………21 Table 2. Estimated Costs……………………………………………………………….........27 List of Attachments Appendix A. Pine Island Ridge Lease 4005……………………………………………... A-1 Appendix B. Property Deeds………….............................................................................. B-1 Appendix C. Pine Island Ridge Improvements………………………………………….. C-1 Appendix D. Conservation Lands within 10 miles of Pine Island Ridge Park………….. D-1 Appendix E. 1948 Aerial Photograph……………………………………………………. E-1 Appendix F. Development Agreement………………………………………………….. F-1 Appendix G. Plant Species Observed at Pine Island Ridge……………………………… G-1 Appendix H. Wildlife Species Observed at Pine Island Ridge ……... …………………. H-1 Appendix -

Nymphalidae, Brassolinae) from Panama, with Remarks on Larval Food Plants for the Subfamily
Journal of the Lepidopterists' Society 5,3 (4), 1999, 142- 152 EARLY STAGES OF CALICO ILLIONEUS AND C. lDOMENEUS (NYMPHALIDAE, BRASSOLINAE) FROM PANAMA, WITH REMARKS ON LARVAL FOOD PLANTS FOR THE SUBFAMILY. CARLA M. PENZ Department of Invertebrate Zoology, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, Wisconsin 53233, USA , and Curso de P6s-Gradua9ao em Biocicncias, Pontiffcia Universidade Cat61ica do Rio Grande do SuI, Av. Ipiranga 6681, FOlto Alegre, RS 90619-900, BRAZIL ANNETTE AIELLO Smithsonian Tropical Research Institute, Apdo. 2072, Balboa, Ancon, HEPUBLIC OF PANAMA AND ROBERT B. SRYGLEY Smithsonian Tropical Research Institute, Apdo. 2072, Balboa, Ancon, REPUBLIC OF PANAMA, and Department of Zoology, University of Oxford, South Parks Road, Oxford, OX13PS, ENGLAND ABSTRACT, Here we describe the complete life cycle of Galigo illioneus oberon Butler and the mature larva and pupa of C. idomeneus (L.). The mature larva and pupa of each species are illustrated. We also provide a compilation of host records for members of the Brassolinae and briefly address the interaction between these butterflies and their larval food plants, Additional key words: Central America, host records, monocotyledonous plants, larval food plants. The nymphalid subfamily Brassolinae includes METHODS Neotropical species of large body size and crepuscular habits, both as caterpillars and adults (Harrison 1963, Between 25 May and .31 December, 1994 we Casagrande 1979, DeVries 1987, Slygley 1994). Larvae searched for ovipositing female butterflies along generally consume large quantities of plant material to Pipeline Road, Soberania National Park, Panama, mo reach maturity, a behavior that may be related as much tivated by a study on Caligo mating behavior (Srygley to the low nutrient content of their larval food plants & Penz 1999). -

Butterfly Gardening Tips & Tricks Gardening for Butterflies Is Fun, Beautiful, and Good for the Environment
Butterfly Gardening Tips & Tricks Gardening for butterflies is fun, beautiful, and good for the environment. It is also simple and can be done in almost any location. The key guidelines are listed below: NO PESTICIDES! Caterpillars are highly susceptible to almost all pesticides so keep them away from your yard if you want butterflies to thrive. Select the right plants. You will need to provide nectar sources for adults and host plants for caterpillars. See the lists below for inspiration. Keep to native varieties as much as possible. Plants come in lots and lots of varieties and cultivars. When selecting plants, especially host plants, try to find native species as close to the natural or wild variety as possible. Provide shelter. Caterpillars need shelter from the sun and shelter from cold nights. Adults need places to roost during the night. And protected areas are needed for the chrysalis to safely undergo its transformation. The best way to provide shelter is with large clumps of tall grasses (native or ornamental) and medium to large evergreen trees and/or shrubs. Nectar Sources Top Ten Nectar Sources: Asclepias spp. (milkweed) Aster spp. Buddleia spp. (butterfly bush) Coreopsis spp. Echinacea spp. (coneflower) Eupatorium spp. (joe-pye weed) Lantana spp. Liatris spp. Pentas spp. Rudbeckia spp. (black-eyed susan) Others: Agastache spp. (hyssop), Apocynum spp. (dogbane), Ceanothus americanus (New Jersey tea), Cephalanthus occidentalis (button bush), Clethra alnifolia, Cuphea spp. (heather), Malus spp. (apple), Mentha spp. (mint), Phlox spp., Pycanthemum incanum (mountain mint), Salivs spp. (sage), Sedum spectabile (stonecrop), Stokesia laevis (cornflower), Taraxacum officinale (dandelion), Triofolium spp. -

Appendix A: Common and Scientific Names for Fish and Wildlife Species Found in Idaho
APPENDIX A: COMMON AND SCIENTIFIC NAMES FOR FISH AND WILDLIFE SPECIES FOUND IN IDAHO. How to Read the Lists. Within these lists, species are listed phylogenetically by class. In cases where phylogeny is incompletely understood, taxonomic units are arranged alphabetically. Listed below are definitions for interpreting NatureServe conservation status ranks (GRanks and SRanks). These ranks reflect an assessment of the condition of the species rangewide (GRank) and statewide (SRank). Rangewide ranks are assigned by NatureServe and statewide ranks are assigned by the Idaho Conservation Data Center. GX or SX Presumed extinct or extirpated: not located despite intensive searches and virtually no likelihood of rediscovery. GH or SH Possibly extinct or extirpated (historical): historically occurred, but may be rediscovered. Its presence may not have been verified in the past 20–40 years. A species could become SH without such a 20–40 year delay if the only known occurrences in the state were destroyed or if it had been extensively and unsuccessfully looked for. The SH rank is reserved for species for which some effort has been made to relocate occurrences, rather than simply using this status for all elements not known from verified extant occurrences. G1 or S1 Critically imperiled: at high risk because of extreme rarity (often 5 or fewer occurrences), rapidly declining numbers, or other factors that make it particularly vulnerable to rangewide extinction or extirpation. G2 or S2 Imperiled: at risk because of restricted range, few populations (often 20 or fewer), rapidly declining numbers, or other factors that make it vulnerable to rangewide extinction or extirpation. G3 or S3 Vulnerable: at moderate risk because of restricted range, relatively few populations (often 80 or fewer), recent and widespread declines, or other factors that make it vulnerable to rangewide extinction or extirpation. -

A Butterfly Injurious to Coconut Palms in British Guiana
CORE ZENODO provided by brought to you by 273 A BUTTERFLY INJURIOUS TO COCONUT PALMS IN BRITISH GUIANA. By LAURENCE D. CLEARE, Jr., F.E.S., Biological Division, Department of Science and Agriculture, British Guiana. (PLATES VIII-X.) During the past year (1914) the coconut palms in the city of Georgetown have been rather severely attacked hy the larvae of the Coconut Butterfly, Brassolis sophorae, L. While this pest has apparently been known in the Colony for some time, it received but little attention until about five years ago, when it made its appearance in the Mahaicony district in very large numbers, causing considerable damage. Mr. F. A. Stockdale, then Assistant Director of Agriculture, investigated the attack and View metadata, citation and similar papers at core.ac.uk reported upon it. From that time until the early part of last year Brassolis was known to most people only by name. In a few months, however, it forced itself upon the attention of the inhabitants, and by June of the same year the result of its ravages was perhaps the most noticeable feature in Georgetown. It was then decided that a census of the coconut palms in the town should be taken and a plan showing the affected areas prepared. The task of preparing this census and plan fell to the writer and it is here proposed to give some description of the work together with notes on the pest. When the work was started there existed no Plant Protection Ordinance in the Colony, though such an ordinance came into force shortly afterwards. -
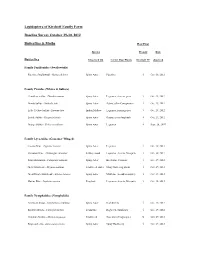
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Butterfly Biodiversity in Singapore with Particular Reference to the Central
Proceedings of the Nature Reserves Survey Seminar. 70re 49(2) (1997) Gardens' Bulletin Singapore 49 (1997) 273-296. ~ laysia and Butterfly Biodiversity in Singapore with Particular :ingapore. Reference to the Central Catchment Nature Reserve discovery, 1 2 ~y Bulletin. S.K. KHEW AND STEVEN S.H. NE0 1103, Tai Keng Gardens, Singapore 535384 re. In: L.M. 2Blk 16, Simei Street 1, #05-13, Melville Park, Singapore 529942 )f Zoology, Abstract Chin, R.T. A total of 381 butterfly species have now been recorded in Singapore of which 18 are new City: Bukit records since 1990. Of this total, 236 species (62%) were recorded during the present JOre. Suppl. survey. A U except 8 (3%) of these occur within the Nature Reserves and 148 (63%) were recorded only within the Nature Reserves. A total of 74 species (31%) within the Reserves were considered very rare. e Nee Soon ion: Marine Introduction l impact of The study of butterflies by amateurs is not new, and indeed, it is through onservation. the observations of these dedicated individuals that much important data have been accumulated over the years. The information on butterfly biodiversity in Singapore is, at most, sketchy. Most of the documentation ater prawn, of the species occurred done during the post-war years until the late 1960s. nidae) from From our literature research, two references stand out: W.A. Fleming's )gy. 43: 299- Butterflies of West Malaysia and Singapore (1991) and Steven Corbet and Maurice Pendlebury's Butterfli es of the Malay Peninsula (1992). Although the latest editions of the two reference books were published in the early ~amalph eops 1990s, most of the updates referred only to the Peninsular Malaysia. -

Wing Pattern Diversity in Brassolini Butterflies (Nymphalidae, Satyrinae)
Wing pattern diversity in Brassolini butterflies (Nymphalidae, Satyrinae) Penz, C.M. & Mohammadi, N. Biota Neotrop. 2013, 13(3): 154-180. On line version of this paper is available from: http://www.biotaneotropica.org.br/v13n3/en/abstract?article+bn03613032013 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v13n3/pt/abstract?article+bn03613032013 Received/ Recebido em 04/01/2013 - Revised/ Versão reformulada recebida em 08/13/2013 - Accepted/ Publicado em 09/13/2013 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. Biota Neotropica is an eletronic journal which is available free at the following site http://www.biotaneotropica.org.br A Biota Neotropica é uma revista eletrônica e está integral e gratuitamente disponível no endereço http://www.biotaneotropica.org.br Biota Neotrop., vol. 13, no. 3 Wing pattern diversity in Brassolini butterflies (Nymphalidae, Satyrinae) Carla Maria Penz1,2 & Neda Mohammadi1 1Department of Biological Sciences, University of New Orleans, 2000 Lakeshore Dr., New Orleans 70148, USA 2Corresponding author: Carla Maria Penz, email: [email protected] PENZ, C.M. -

Family LYCAENIDAE: 268 Species GOSSAMERWINGS
Family LYCAENIDAE: 268 species GOSSAMERWINGS Subfamily Miletinae: 1 (hypothetical) species Harvesters Feniseca tarquinius tarquinius Harvester Hypothetical, should occur in N Tamaulipas, but currently unknown from Mexico Subfamily Lycaeninae: 6 species Coppers Iophanus pyrrhias Guatemalan Copper Lycaena arota arota Tailed Copper Lycaena xanthoides xanthoides Great Copper Lycaena gorgon gorgon Gorgon Copper Lycaena helloides Purplish Copper Lycaena hermes Hermes Copper Subfamily Theclinae: 236 species Hairstreaks Tribe Theclini: 3 species Hairstreaks Hypaurotis crysalus crysalus Colorado Hairstreak Habrodais grunus grunus Golden Hairstreak verification required for Baja California Norte Habrodais poodiae Baja Hairstreak Tribe Eumaeini: 233 Hairstreaks Eumaeus childrenae Great Cycadian (= debora) Eumaeus toxea Mexican Cycadian Theorema eumenia Pale-tipped Cycadian Paiwarria antinous Felders' Hairstreak Paiwarria umbratus Thick-tailed Hairstreak Mithras sp. undescribed Pale-patched Hairstreak nr. orobia Brangas neora Common Brangas Brangas coccineifrons Black-veined Brangas Brangas carthaea Green-spotted Brangas Brangas getus Bright Brangas Thaeides theia Brown-barred Hairstreak Enos thara Thara Hairstreak Enos falerina Falerina Hairstreak Evenus regalis Regal Hairstreak Evenus coronata Crowned Hairstreak Evenus batesii Bates’ Hairstreak Atlides halesus corcorani Great Blue Hairstreak Atlides gaumeri White-tipped Hairstreak Atlides polybe Black-veined Hairstreak Atlides inachus Spying Hairstreak Atlides carpasia Jeweled Hairstreak Atlides -

Specimen Records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895
Catalog: Oregon State Arthropod Collection 2019 Vol 3(2) Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895 Jon H. Shepard Paul C. Hammond Christopher J. Marshall Oregon State Arthropod Collection, Department of Integrative Biology, Oregon State University, Corvallis OR 97331 Cite this work, including the attached dataset, as: Shepard, J. S, P. C. Hammond, C. J. Marshall. 2019. Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895. Catalog: Oregon State Arthropod Collection 3(2). (beta version). http://dx.doi.org/10.5399/osu/cat_osac.3.2.4594 Introduction These records were generated using funds from the LepNet project (Seltmann) - a national effort to create digital records for North American Lepidoptera. The dataset published herein contains the label data for all North American specimens of Lycaenidae and Riodinidae residing at the Oregon State Arthropod Collection as of March 2019. A beta version of these data records will be made available on the OSAC server (http://osac.oregonstate.edu/IPT) at the time of this publication. The beta version will be replaced in the near future with an official release (version 1.0), which will be archived as a supplemental file to this paper. Methods Basic digitization protocols and metadata standards can be found in (Shepard et al. 2018). Identifications were confirmed by Jon Shepard and Paul Hammond prior to digitization. Nomenclature follows that of (Pelham 2008). Results The holdings in these two families are extensive. Combined, they make up 25,743 specimens (24,598 Lycanidae and 1145 Riodinidae).