Discovery of Yttrium, Zirconium, Niobium, Technetium, and Ruthenium Isotopes A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Production of Radionuclides
CHAPTER 15 Production of Radionuclides Contents 15.1. General considerations 389 15.2. Irradiation yields 390 15.3. Second-order reactions 393 15.4. Target considerations 397 15.4.1. Physical properties 397 15.4.2. Chemical properties 398 15.5. Product specifications 399 15.5.1. Radiochemical processing 399 15.5.2. Specific activity 400 15.5.3. Labeling 400 15.5.4. Radiochemical purity 401 15.6. Recoil separations 402 15.6.1. Target recoil products 402 15.6.2. Hot atom reactions 403 15.6.3. The Szilard-Chalmers process 404 15.7. Fast radiochemical separations 406 15.7.1. Production of 11C labeled compounds 407 15.7.2. Auto-batch procedures 407 15.7.3. On-line procedures: gas-phase separation 409 15.7.4. On-line procedures: solvent extraction 412 15.7.5. Mass separator procedures 412 15.8. Exercises 412 15.9. Literature 414 This chapter discusses production of radionuclides for beneficial use in science, medicine and technology. The nuclear fundamentals for the production processes have been given in Chapters 11 to 14. The formation of radionuclides is discussed in several Chapters: e.g. cosmogenic reactions leading to the formation of short-lived radionuclides in nature (Ch. 5 and 10); thermonuclear reactions leading to the formation of long-lived radioactivity in the universe (Ch. 17); the synthesis of trans-uranium elements (Ch. 16 and 19-21). The production and isolation of separated fission products is treated separately (Ch. 19-21). This chapter discusses aspects of fundamental importance to the production of radionuclides by a variety of methods. -

|||||||||||||III USOO5302369A United States Patent (19) (11) Patent Number: 5,302,369 Day Et Al
|||||||||||||III USOO5302369A United States Patent (19) (11) Patent Number: 5,302,369 Day et al. (45) Date of Patent: k Apr.p 12, 1994 (54) MERSHERES FOR RADIATION OTHER PUBLICATIONS RAPY Makishima et al., "Elastic Moduli and Refractive Indi (75) Inventors: Delbert E. Day, Rolla; Gary J. ces of Aluminosilicate Glasses Containing Y2O3, La2O3, Ehrhardt, Columbia, both of Mo. and TiO2'; Journal of the American Ceramic Society; 73) Assignee: The Curators of the University of vol. 61, pp. 247-249; May-Jun. 1978. Missouri, Columbia, Mo. Loehman, "Preparation and Properties of Yttri (*) Notice: The portion of the term of this patent um-Silicon-Aluinum Oxynitride Glasses"; Journal of subsequent to Dec. 6, 2005 has been the American Ceramic Society; vol. 62, pp. 491-494; disclaimed. Sep.-Oct. 1979. Makishima, et al., “Alkaline Durability of High Elastic R21 Appl.pp No.:O 751,721 Modulus Alumino-Silicatey s Glasses Containing Y2O3, (22 Filed: Aug. 29, 1991 La2O3 and TiO2'; Journal of Non-Crystalline Solids 38 & 39, pp. 661-666 (1980). Related U.S. Application Data Bonder, et al., "Phase Equilibria in the System Y2O (63) Stylist Ser. No. 280,005, FS, 59. al 3-Al2O3-SiO2'; I. V. Grebenschikov Institute of Sili Oe W1c is a contation o er. O. is aws's cate Chemistry, Academy of Sciences, USSR, trans Nov. 19, 1984, Pat. No. 4,789,501. lated from Izvestiya Akademii Nauk USSR, Seriya 51 int. Cl. ....................... A61K 43/00; A61N 5/00; Khimicheskaya, No. 7, pp. 1325-1326, Jul. 1964. CO3C 3/095; CO3C 3/097 52 U.S. -

Lawrence Berkeley National Laboratory Recent Work
Lawrence Berkeley National Laboratory Recent Work Title RADIOCHEMICAL AND SPECTROMETER STUDIES OF SEVERAL NEUTRON-DEFICIENT ZIRCONIUM ISOTOPES AND THEIR DECAY PRODUCTS Permalink https://escholarship.org/uc/item/5ck8q7xw Authors Hyde, Earl K. O'Kelley, Grover D. Publication Date 1950-12-28 eScholarship.org Powered by the California Digital Library University of California UCRL- .Jt)AJ,-.64.. _.; >-w .J w ... ~ ,• 0:: w m I . ;•· <( z 0:: 0 LL ..J u<( LL 0 >- 1-- TWO-WEEK LOAN COPY (/) This is a Library Circulating Copy 0:: which may be borrowed for two weeks. w For a personal retention copy, call > Tech. Info. Diuision, Ext. 5545 z '.-o:-. :J ~~ RADIATION LABORATORY DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any · information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. -

Calculation of Neutron Cross Sections on Isotopes of Yttrium and Zirconium
pp. LA-7789-MS Informal Report Calculation of Neutron Cross Sections on Isotopes of Yttrium and Zirconium co O CO 5 • . LOS ALAMOS SCIENTIFIC LABORATORY Rpst Office Bex 1663 Los Alamos. New Mexico 37545 A LA-7789-MS Informal Report UC-34c Issued: April 1979 Calculation of Neutron Cross Sections on Isotopes of Yttrium and Zirconium E. D. Arthur - NOTICE- Tim report wt piepited u an account of work sponsored by the United Stales Government. Neither the United States nor the United Statci Department of Energy, nor any of their empioyeet, nor any of their contractor*, subcontractor!, or their employees, nukes any warranty, express or implied, ot astumes any legal liability 01 responsibility foi the accuiacy, completeness or luefulnets of any Information, apparatus, product or piocett ductoied.oi ^presents that iti uie would not infringe privately owned rights. CALCULATION OF NEUTRON CROSS SECTIONS ON ISOTOPES OF YTTRIUM AND ZIRCONIUM by E. D. Arthur ABSTRACT Multistep Hauser-Feshbach calculations with preequilibrium corrections have been made for neutron-induced reactions on yttrium and zirconium isotopes between 0.001 and 20 MeV. Recent- ly new neutron cross-section data have been measured for unstable isotopes of these elements. These data, along with results from charged-particle simulation of neutron reactions, provide unique opportunities under which to test nuclear-model techniques and parameters in this mass region. We have performed a complete and consistent analysis of varied neutron reaction types using input parameters determined independently from additional neutron and charged-particle data. The overall agreement between our calcula- tions and a wide variety of experimental results available for these nuclei leads to increased confidence in calculated cross sections made where data are incomplete or lacking. -

Technische Universität München Physik-Department Study of The
Technische Universität München Physik-Department Study of the biokinetics of zirconium isotopes in humans and its relevance to internal dosimetry Matthias Greiter Vollständiger Abdruck der von der Fakultät für Physik der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. J. L. van Hemmen Prüfer der Dissertation: 1. Hon.-Prof. Dr. H.G. Paretzke 2. Univ.-Prof. Dr. F. von Feilitzsch Die Dissertation wurde am 27.09.2007 bei der Technischen Universität München eingereicht und durch die Fakultät für Physik am 22.04.2008 angenommen. i Author contact information: Matthias Greiter Institute of Radiation Protection Helmholtz Zentrum München German Research Center for Environmental Health (GmbH) Ingolstaedter Landstrasse 1 85764 Neuherberg Germany E-mail: [email protected] ii Table of contents Abstract ...................................................................................................................................... 1 List of acronyms, symbols and abbreviations ............................................................................ 2 1 Aim of the study......................................................................................................... 3 1.1 Natural occurrence of zirconium compounds ............................................................ 3 1.2 Applications of stable zirconium................................................................................ 5 1.3 Applications -

Development of a National Neutron Database for Nuclear Technology
- 7 - XA9744992 DEVELOPMENT OF A NATIONAL NEUTRON DATABASE FOR NUCLEAR TECHNOLOGY A. V. Ignatyuk, V.N. Kononov, B.D. Kuzminov, V.N. Manokhin M.N. Nikolaev, B.I. Furzov State Research Centre of the Russian Federation Institute of Physics and Power Engineering, Obninsk Abstract This paper describes the stages of a many years activities at the IPPE consisting of the measurement, theoretical description and evaluation of neutron data, and of the establishment of a national data bank of neutron data for nuclear technology. A list of libraries which are stored at the Nuclear Data Centre is given. Introduction Currently, nuclear technology has found wide applications in a number of fields in science and technology consisting of: - nuclear energy (nuclear fission reactors, nuclear fission reactors, radioisotopic sources) - nuclear transmutation (production of radioisotopes for medical and other applications, burn-up of long-lived radio nuclides, radiation alloying of semiconducting materials, etc..) - nuclear instrumentation in industry (activation analysis, nuclear micro-analysis, control of fissile materials, etc..) - scientific investigations (nuclear physics, nuclear astrophysics, medicine, radiobiology, agro-radiology, ecology, etc.). In the sphere of nuclear technology, a number of processes use more than 500 stable and radioactive nuclides in a broad energy range. To ascertain the proper functioning of nuclear installations which have various functions and applications in industrial projects and scientific investigations, there is a need for nuclear data that characterize the properties of nuclear reactions. The reliability of radiation and nuclear safety, the ecological acceptance, and the economical planning of nuclear installations, all depend on the completeness, quality and accuracy of nuclear data. -
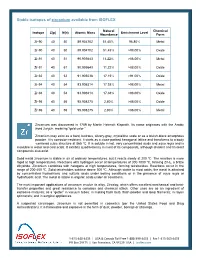
Stable Isotopes of Zirconium Available from ISOFLEX
Stable isotopes of zirconium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Zr-90 40 50 89.904702 51.45% 96.80% Metal Zr-90 40 50 89.904702 51.45% >98.00% Oxide Zr-91 40 51 90.905643 11.22% >88.00% Metal Zr-91 40 51 90.905643 11.22% >88.00% Oxide Zr-92 40 52 91.905038 17.15% >91.00% Oxide Zr-94 40 54 93.906314 17.38% >98.00% Metal Zr-94 40 54 93.906314 17.38% >98.00% Oxide Zr-96 40 56 95.908275 2.80% >86.00% Oxide Zr-96 40 56 95.908275 2.80% >86.00% Metal Zirconium was discovered in 1789 by Martin Heinrich Klaproth. Its name originates with the Arabic word zargun, meaning “gold color.” Zirconium may exist as a hard, lustrous, silvery-gray, crystalline scale or as a bluish-black amorphous powder. It is corrosion-resistant. It starts as a close-packed hexagonal lattice and transforms to a body -centered cubic structure at 865 ºC. It is soluble in hot, very concentrated acids and aqua regia and is insoluble in water and cold acids. It exhibits quadrivalency in most of its compounds, although divalent and trivalent compounds also exist. Solid metal zirconium is stable in air at ordinary temperatures, but it reacts slowly at 200 ºC. The reaction is more rapid at high temperatures. Reactions with hydrogen occur at temperatures of 300-1000 ºC, forming ZrH2, a brittle dihydride. Zirconium combines with halogens at high temperatures, forming tetrahalides. -

Isotope Shifts from Collinear Laser Spectroscopy of Doubly Charged Yttrium Isotopes
This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Author(s): Vormawah, L. J.; Vilén, Markus; Beerwerth, R.; Campbell, P.; Cheal, B.; Dicker, A.; Eronen, Tommi; Fritzsche, S.; Geldhof, Sarina; Jokinen, Ari; Kelly, S.; Moore, Iain; Reponen, Mikael; Rinta-Antila, Sami; Stock, S. O.; Voss, Annika Title: Isotope shifts from collinear laser spectroscopy of doubly charged yttrium isotopes Year: 2018 Version: Please cite the original version: Vormawah, L. J., Vilén, M., Beerwerth, R., Campbell, P., Cheal, B., Dicker, A., Eronen, T., Fritzsche, S., Geldhof, S., Jokinen, A., Kelly, S., Moore, I., Reponen, M., Rinta- Antila, S., Stock, S. O., & Voss, A. (2018). Isotope shifts from collinear laser spectroscopy of doubly charged yttrium isotopes. Physical Review A, 97(4), Article 042504. https://doi.org/10.1103/PhysRevA.97.042504 All material supplied via JYX is protected by copyright and other intellectual property rights, and duplication or sale of all or part of any of the repository collections is not permitted, except that material may be duplicated by you for your research use or educational purposes in electronic or print form. You must obtain permission for any other use. Electronic or print copies may not be offered, whether for sale or otherwise to anyone who is not an authorised user. PHYSICAL REVIEW A 97, 042504 (2018) Isotope shifts from collinear laser spectroscopy of doubly charged yttrium isotopes L. J. Vormawah,1 M. Vilén,2 R. Beerwerth,3,4 P. Campbell,5 B. Cheal,1,* A. Dicker,5 T. Eronen,2 S. -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Periodic Table of Elements
The origin of the elements – Dr. Ille C. Gebeshuber, www.ille.com – Vienna, March 2007 The origin of the elements Univ.-Ass. Dipl.-Ing. Dr. techn. Ille C. Gebeshuber Institut für Allgemeine Physik Technische Universität Wien Wiedner Hauptstrasse 8-10/134 1040 Wien Tel. +43 1 58801 13436 FAX: +43 1 58801 13499 Internet: http://www.ille.com/ © 2007 © Photographs of the elements: Mag. Jürgen Bauer, http://www.smart-elements.com 1 The origin of the elements – Dr. Ille C. Gebeshuber, www.ille.com – Vienna, March 2007 I. The Periodic table............................................................................................................... 5 Arrangement........................................................................................................................... 5 Periodicity of chemical properties.......................................................................................... 6 Groups and periods............................................................................................................. 6 Periodic trends of groups.................................................................................................... 6 Periodic trends of periods................................................................................................... 7 Examples ................................................................................................................................ 7 Noble gases ....................................................................................................................... -

The Use of Yttrium in Medical Imaging and Therapy: Historical Background Cite This: Chem
Chem Soc Rev View Article Online TUTORIAL REVIEW View Journal | View Issue The use of yttrium in medical imaging and therapy: historical background Cite this: Chem. Soc. Rev., 2020, 49,6169 and future perspectives Ben J. Tickner, a Graeme J. Stasiuk, b Simon B. Duckett a and Goran Angelovski *c Yttrium is a chemically versatile rare earth element that finds use in a range of applications including lasers and superconductors. In medicine, yttrium-based materials are used in medical lasers and biomedical implants. This is extended through the array of available yttrium isotopes to enable roles for 90Y complexes as radiopharmaceuticals and 86Y tracers for positron emission tomography (PET) imaging. The naturally abundant isotope 89Y is proving to be suitable for nuclear magnetic resonance Received 11th April 2020 investigations, where initial reports in the emerging field of hyperpolarised magnetic resonance imaging DOI: 10.1039/c9cs00840c (MRI) are promising. In this review we explore the coordination and radiochemical properties of Creative Commons Attribution 3.0 Unported Licence. yttrium, and its role in drugs for radiotherapy, PET imaging agents and perspectives for applications in rsc.li/chem-soc-rev hyperpolarised MRI. Key learning points 1. Versatility of yttrium coordination chemistry results in a vast number of complexes with variable physicochemical features. 2. The properties of a range of yttrium isotopes enable their use in radiochemistry. 3. Yttrium radioisotopes exploited for medical imaging and radiotherapy applications. 4. Suitability of yttrium for NMR investigations and new perspectives for yttrium-based hyperpolarized MRI. This article is licensed under a Open Access Article. Published on 23 July 2020. -

Theoretical Potential of the CANDU Core for Transmutation of PWR TRU Nuclides
Proceedings of the Reactor Physics Asia 2015 (RPHA15) Conference Jeju, Korea, Sept. 16-18, 2015 Theoretical Potential of the CANDU Core for Transmutation of PWR TRU Nuclides Jinqi Lyu, Donny Hartanto, Mohammad Abdul Motalab and Yonghee Kim Department of Nuclear and Quantum Engineering, KAIST 291 Daehak-ro, Yusong-gu, Daejeon 34141, Republic of Korea *Corresponding author: [email protected] 1. Introduction 2. Analysis Models and Methods Transuranic (TRU) nuclides generated in spent fuels In this work, the analysis is done only for a 2-D fuel from the Light Water Reactor (LWR) pose a big assembly lattice because the core characteristics can be challenge in nuclear waste management due to its well determined through lattice analyses in CANDU potential diversion, high radiological toxicity and long core. The lattice calculation is performed for a lifetime. One of the potential approaches for dealing CANFLEX fuel bundle, as illustrated in Fig. 1, which with spent nuclear fuel is to fully utilize (transmute) the can improve the operating and safety margin, as well as TRU nuclides in nuclear reactors as nuclear fuels. the economics of a CANDU reactor. There have been many studies about utilizing TRU nuclides in CANDU reactors since many design features of CANDU reactor make it uniquely suitable for transmuting TRU nuclides, such as simple fabrication and handling of active fuels, precise management of core reactivity, high neutron economy and so on [1]. The concept of MOX (mixed oxides) fuel, which mixes actinides with natural uranium, and IMF (inert matrix fuel), which mixes actinides with silicon carbide, were proposed previously.