Guide to Local Production – WHO-Recommended Handrub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Who, Whom, Whoever, and Whomever
San José State University Writing Center www.sjsu.edu/writingcenter Written by Cassia Homann Who, Whom, Whoever, and Whomever People often do not know when to use the pronouns “who,” “whom,” “whoever,” and “whomever.” However, with a simple trick, they will always choose the correct pronoun. For this trick, use the following key: who = she, he, I, they whom = her, him, me, them Who In the following sentences, use the steps that are outlined to decide whether to use who or whom. Example Nicole is a girl (who/whom) likes to read. Step 1: Cover up the part of the sentence before “who/whom.” Nicole is a girl (who/whom) likes to read. Step 2: For the remaining part of the sentence, test with a pronoun using the above key. Replace “who” with “she”; replace “whom” with “her.” Who likes to read = She likes to read Whom likes to read = Her likes to read Step 3: Consider which one sounds correct. (Remember that the pronoun “she” is the subject of a sentence, and the pronoun “her” is part of the object of a sentence.) “She likes to read” is the correct wording. Step 4: Because “she” works, the correct pronoun to use is “who.” Nicole is a girl who likes to read. Who, Whom, Whoever, and Whomever, Fall 2012. Rev. Summer 2014. 1 of 4 Whom Example Elizabeth wrote a letter to someone (who/whom) she had never met. Step 1: Cover up the part of the sentence before “who/whom.” Elizabeth wrote a letter to someone (who/whom) she had never met. -

Case Management and Staff Support Across NCI States
What the 2018-19 NCI® Child Family Survey data tells us about Case Management and Staff Support Across NCI States This report tells us about: • What NCI tells us about case management and staff support • Why this is important What is NCI? Each year, NCI asks people with intellectual and developmental disabilities (IDD) and their families how they feel about their lives and the services they get. NCI uses surveys so that the same questions can be asked to people in all NCI states. Who answered questions to this survey? Questions for this survey are answered by a person who lives in the same house as a child who is getting services from the state. Most of the time, a parent answers these questions. Sometimes a sibling or someone who lives with the child and knows them well answers these questions. 2 How are data shown in this report? NCI asks questions about planning services and supports for children who get services from the state. In this report we see how family members of children getting services answered questions about planning services and supports. • In this report, when we say “you” we mean the person who is answering the question (most of the time, a parent). • In this report, when we say “child” we mean the child who is getting services from the state. 3 We use words and figures to show the number of yes and no answers we got. Some of our survey questions have more than a yes or no answer. They ask people to pick: “always,” “usually,” “sometimes,” or “seldom/never.” For this report, we count all “always” answers as yes. -

The Function of Phrasal Verbs and Their Lexical Counterparts in Technical Manuals
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1991 The function of phrasal verbs and their lexical counterparts in technical manuals Brock Brady Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Applied Linguistics Commons Let us know how access to this document benefits ou.y Recommended Citation Brady, Brock, "The function of phrasal verbs and their lexical counterparts in technical manuals" (1991). Dissertations and Theses. Paper 4181. https://doi.org/10.15760/etd.6065 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF THE THESIS OF Brock Brady for the Master of Arts in Teaching English to Speakers of Other Languages (lESOL) presented March 29th, 1991. Title: The Function of Phrasal Verbs and their Lexical Counterparts in Technical Manuals APPROVED BY THE MEMBERS OF THE THESIS COMMITTEE: { e.!I :flette S. DeCarrico, Chair Marjorie Terdal Thomas Dieterich Sister Rita Rose Vistica This study investigates the use of phrasal verbs and their lexical counterparts (i.e. nouns with a lexical structure and meaning similar to corresponding phrasal verbs) in technical manuals from three perspectives: (1) that such two-word items might be more frequent in technical writing than in general texts; (2) that these two-word items might have particular functions in technical writing; and that (3) 2 frequencies of these items might vary according to the presumed expertise of the text's audience. -

Grammar Worksheets: Who Or Whom?
Grammar Worksheets: Who or Whom? http://www.grammar-worksheets.com People are so mystified (confused) about the use of who and whom that some of us are tempted to throw RXUKDQGVLQWKHDLUDQGVD\³LWMXVWGRHVQ¶WPDWWHU´%XWLWGRHVPDWWHU7KRVHZKRNQRZ DQGQRWMXVW English teachers), judge those who misuse it. Not using who and whom correctly can cost you, not just in schooOEXWDOVRLQOLIH/HW¶VJHWLWGRZQQRZ Who and Whom are Pronouns 7KDW¶VULJKW who and whom are pronouns. And if you recall, a pronoun is a word that takes the place of a noun. Sometimes we use pronouns instead of nouns. :HZRXOGQRWVD\³-HVVH GRHVQ¶WOLNHWKHSULQFLSDO0V7KRPDVZDVKLUHGDWKLVVFKRRO´7KHQDPHMs. Thomas LVDQRXQ)RUWKLVVHQWHQFHWRIORZZHZRXOGZULWH³-HVVHGRHVQ¶WOLNHWKHSULQFLSDOZKRZDV KLUHGDWKLVVFKRRO´ It All Depends on Case In English grammar, we have a term called case, which refers to pronouns. The case of a pronoun can be either subject or object, depending on its use in a sentence. Take a look at this table. Subject Object I me he him she her we us they them who whom The pronoun who is used as a subject; whom is used as an object. Who used correctly: Janice is the student who has read the most books. Whom used correctly: Janice is the student whom the teachers picked as outstanding. How Can I Determine Which One to Use? Break up the sentence into two parts. Janice is the student. She (Janice) has read the most books. Janice is the student. The teachers picked her (Janice) as outstanding. If you use I, he, she, we, or they, then the correct form is who. If you use me, him, her, us, or them, then the correct form is whom. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -

Bio-Butanol Production from Glycerol with Clostridium
Lin et al. Biotechnol Biofuels (2015) 8:168 DOI 10.1186/s13068-015-0352-6 RESEARCH Open Access Bio‑butanol production from glycerol with Clostridium pasteurianum CH4: the effects of butyrate addition and in situ butanol removal via membrane distillation De‑Shun Lin1, Hong‑Wei Yen2, Wei‑Chen Kao1, Chieh‑Lun Cheng1, Wen‑Ming Chen3, Chieh‑Chen Huang4 and Jo‑Shu Chang1,4,5* Abstract Background: Clostridium pasteurianum CH4 was used to produce butanol from glycerol. The performance of butanol fermentation was improved by adding butyrate as the precursor to trigger the metabolic pathway toward butanol production, and by combining this with in situ butanol removal via vacuum membrane distillation (VMD) to avoid the product inhibition arising from a high butanol concentration. 1 Results: Adding 6 g L− butyrate as precursor led to an increase in the butanol yield from 0.24 to 0.34 mol butanol 1 (mol glycerol)− . Combining VMD and butyrate addition strategies could further enhance the maximum effective 1 1 butanol concentration to 29.8 g L− , while the yield was also improved to 0.39 mol butanol (mol glycerol)− . The butanol concentration in the permeate of VMD was nearly five times higher than that in the feeding solution. Conclusions: The proposed butyrate addition and VMD in situ butanol removal strategies are very effective in enhancing both butanol titer and butanol yield. This would significantly enhance the economic feasibility of fermen‑ tative production of butanol. The VMD-based technology not only alleviates the inhibitory effect of butanol, but also markedly increases butanol concentration in the permeate after condensation, thereby making downstream process‑ ing easier and more cost-effective. -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -
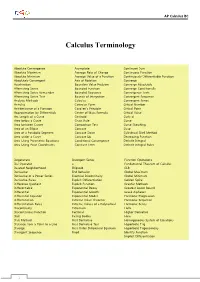
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

A Cooked Composition of Glycerine and Hydrogenated Starch Hydrolysate and the Use Thereof As a Stabilizer for L-Aspartic Acid Sweetening Agent in Comestibles
Europaisches Patentamt 0 323 442 J> European Patent Office Publication number: A1 Office europeen des brevets EUROPEAN PATENT APPLICATION @ Application number: 89102633.8 IntCI* A 23 L 1/236 A 23 G 3/30 @ Date of filing: 27.03.86 2g) Priority: 29.03.85 US 717630 © Applicant: NABISCO BRANDS, INC. 100 DeForest Avenue East Hanover New Jersey 07936 (US) §) Date of publication of application: 05.07.89 Bulletin 89/27 @ Inventor: Carroll, Thomas J. States: BE DE FR GB IT 20-30 43rd Street g) Designated Contracting Astoria, N.Y. (US) jig) Publication number of the earlier application in Kehoe, Gary S. accordance with Art. 76 EPC: 0 196 640 10 Cross Hill Road Ridgefield, Conn. (US) @) Representative : Brauns, Hans-Adolf, Dr. rer. nat. et al Hoffmann, Eitle & Partner, Patentanwalte Arabellastrasse 4 D-8000 Munich 81 (DE) @ A cooked composition of glycerine and hydrogenated starch hydrolysate and the use thereof as a stabilizer for L-aspartic acid sweetening agent in comestibles. (g) A cooked composition of glycerine and hydrogenated starch hydrolysate having a moisture content of 10 ± 6% (W/W). Said composition can be used as a stabilizer for L-aspartic acid sweetening agent in comestibles. CM CO CM eo a. LU Bundesdruckerei Berlin EP 0 323 442 A1 Description A COOKED COMPOSITION OF GLYCERINE AND HYDROGENATED STARCH HYDROLYSATE AND THE USE THEREOF AS A STABILIZER FOR L-ASPARTIC ACID SWEETENING AGENT IN COMESTIBLES Aspartame which is used extensively in many types of sugarless foodstuffs, or other comestible products, 5 such as chewing gum, is known to readily decompose in the presence of moisture into decomposition products such as diketopiperizine which causes a significant loss in the sweetness properties of such products during their shelf lives. -

Electronic Nicotine Delivery Device (ENDS)"
Qeios, CC-BY 4.0 · Review, March 4, 2020 Review of "Electronic Nicotine Delivery Device (ENDS)" Clive Bates Overall. T he definition provided is helpful but there are a few remaining ambiguities, most notably with respect to the inclusion or exclsuion of heated tobacco products within this definition. I think the set of definitions in this field should be looked at together for coherence, and speculate whether they could be built from a set of elemental variables. Device? Strictly, ENDS are not devices, but device-liquid combinations. It is possible to put a non-nicotine liquid in a refillable vaping device that is also designed for nicotine (i.e a flavoured e-liquid with no nicotine). So a device only becomes an ENDS when paired with liquid or class of liquids that contain nicotine. T he same hardware is not an ENDS if not using a nicotine liquid. Not all devices are hand-held: there are electronic hookah pipes using nicotine liquids, for example, that stand on a table or the floor. H umectant? T he function of the propylene glycol or glycerol in the e-liquid is not primarily as a humectant but as: (1) a diluent (to create a chosen nicotine concentration) and, (2) an excipient (a inert carrier for the active ingredients - flavours and nicotine). While these agents can also be humectants, that is not their primary function in ENDS. Clarity over excipient names. T here is great confusion about the names of excipients and an opportunity for clarity here: "...one or more excipients, which may include propylene glycol (PG), glycerol or other excipients. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance.