Massachusetts Division of Fisheries & Wildlife
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

2015 Summary of Changes to Endangered, Threatened, And
2015 Update to State Listed Species The Department of Energy and Environmental Protection (DEEP) is required to review, at least every five years, the designation of species as endangered, threatened, or of special concern to determine whether species should be: (1) added or removed from the list; or, if necessary, (2) change the designation from one category to another. The following is a summary of the changes to the State Endangered Species list (DEEP Regulations Sections 26‐306‐4, 26‐306‐5, and 26‐306‐6) that became effective on August 5, 2015. The complete list can be found on the DEEP website. Summary of Amphibian Changes New species added Necturus maculosus, Mudpuppy added as Special Concern Summary of Reptile Changes New species added Clemmys guttata, Spotted turtle added as Special Concern Malaclemys terrapin terrapin, Northern diamondback terrapin added as Special Concern Taxonomic Changes Eumeces fasciatus, Five‐lined skink changed to Plestiodon fasciatus Liochlorophis vernalis, Smooth green snake changed to Opheodrys vernalis Summary of Bird Changes Northern diamondback terrapin Status Changes Falco sparverius, American kestrel downlisted to Special Concern Progne subis, Purple martin downlisted to Special Concern Sturnella magna, Eastern meadowlark uplisted to Threatened New species added Accipiter gentilis, Northern goshawk added as Threatened Setophaga cerulea, Cerulean warbler added as Special Concern Species delisted Anas discors, Blue‐winged teal Laterallus jamaicensis, Black rail Cerulean warbler Taxonomic changes Parula americana, Northern parula changed to Setophaga americana 1 Summary of Mammal Changes Status Changes Myotis leibii, Eastern small‐footed bat uplisted to Endangered New Species Added Myotis lucifugus, Little brown bat added as Endangered Myotis septentrionalis, Northern long‐eared bat added as Endangered (also Federally Threatened) Perimyotis subflavus, Tri‐colored bat added as Endangered Taxonomic Changes Phocoena phocoena, Harbor porpoise changed to Phocoena Northern long‐eared bat phocoena ssp. -

Lepidoptera, Noctuidae, Xyleninae)
Zootaxa 3755 (2): 165–178 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3755.2.3 http://zoobank.org/urn:lsid:zoobank.org:pub:6E3EB860-21C2-4F44-816E-DCCDAB6ECB0D A revision of the genus Protarchanara Beck, 1999 with description of a new genus and three new species (Lepidoptera, Noctuidae, Xyleninae) ANTON V. VOLYNKIN1, 5, ALEXEJ YU. MATOV2, PÉTER GYULAI3 & GOTTFRIED BEHOUNEK4 1Altai State University, Lenina str., 61, RF-656000, Barnaul, Russia; Tigirek State Natural Reserve, office 42, Nikitina str. 111, RF- 656043, Barnaul, Russia. E-mail: [email protected] 2Zoological Institute RAS, Universitetskaja emb. 1, RF-199034, Saint-Petersburg, Russia. E-mail: [email protected], [email protected] 3H-3530 Miskolc, Mélyvölgy 13/A, Hungary. E-mail: [email protected] 4Sudetenstrasse 6, В-85567 Graphing bei München, Germany. E-mail: [email protected] 5Corresponding author Abstract The genus Protarchanara Beck, 1999 is revised. The new genus Hydredes gen. n. (type species Hydredes yakobsoni sp. n.) is described. Three new species, Hydredes yakobsoni sp. n., H. shchetkini sp. n. and Protarchanara mythimnoida sp. n. are described from Central Asia. Two species, Arenostola delattini Wiltshire, 1953 and Hydraecia praecipua Hacker & Nekrasov, 2001 are transferred to the genus Hydredes gen. n. Protarchanara brevilinea impudica (Staudinger, 1892) stat. n. is upgraded to subspecific level. Lectotypes for Namangana contumax Püngeler, 1902, Sidemia (Luperina) johni Püngeler, 1914 and Nonagria impudica Staudinger, 1892 are designated. The adults and male and female genitalia are il- lustrated. -

Errata and First Update to the 2010 Checklist of the Lepidoptera Of
Errata and first uppppdate to the 2010 checklist of the Lepidoptera of Alberta Gregory R. Pohl, Jason J Dombroskie, Jean‐François Landry, Charles D Bird, and Vazrick Nazari lead author contact: [email protected] Introduction: Since the Annotated list of the Lepidoptera of Alberta was published in March 2010 (Pohl et al. 2010), a few typographical and nomenclatural errors have come to the authors' attention, as well as three erroneous AB records that were inadvertently omitted from that publication. Additionally, a considerable number of new AB species records have been brought to our attention since that checklist went to press. As expected, most are microlepidoptera. We detail all these items below, in what we hope will be a regular series of addenda to the AB list. If you are aware of further errors or additions to the AB Lepidoptera list, please contact the authors. Wit hin the NidNoctuoidea, there are a few minor iiiinconsistencies in the order of species wihiithin genera, and in the order of genera within tribes or subtribes, as compared to the sequence published by Lafontaine & Schmidt (2010). As well, the sequence of tribes in the AB list does not exactly match that of Lafontaine & Schmidt (2010), particularly in the Erebinae. We are not detailing those minor differences here unless they involve a move to a new genus or new higher taxonomic category. Errata: Abstract, p. 2, line 10, should read "1530... annotations are given" 41 Nemapogon granella (p. 55). Add Kearfott (1905) to the AB literature records. 78 Caloptilia syringella (p. 60). This species should be placed in the genus Gracillaria as per De Prins & De Prins (2005). -

Check List of Noctuid Moths (Lepidoptera: Noctuidae And
Бiологiчний вiсник МДПУ імені Богдана Хмельницького 6 (2), стор. 87–97, 2016 Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 6 (2), pp. 87–97, 2016 ARTICLE UDC 595.786 CHECK LIST OF NOCTUID MOTHS (LEPIDOPTERA: NOCTUIDAE AND EREBIDAE EXCLUDING LYMANTRIINAE AND ARCTIINAE) FROM THE SAUR MOUNTAINS (EAST KAZAKHSTAN AND NORTH-EAST CHINA) A.V. Volynkin1, 2, S.V. Titov3, M. Černila4 1 Altai State University, South Siberian Botanical Garden, Lenina pr. 61, Barnaul, 656049, Russia. E-mail: [email protected] 2 Tomsk State University, Laboratory of Biodiversity and Ecology, Lenina pr. 36, 634050, Tomsk, Russia 3 The Research Centre for Environmental ‘Monitoring’, S. Toraighyrov Pavlodar State University, Lomova str. 64, KZ-140008, Pavlodar, Kazakhstan. E-mail: [email protected] 4 The Slovenian Museum of Natural History, Prešernova 20, SI-1001, Ljubljana, Slovenia. E-mail: [email protected] The paper contains data on the fauna of the Lepidoptera families Erebidae (excluding subfamilies Lymantriinae and Arctiinae) and Noctuidae of the Saur Mountains (East Kazakhstan). The check list includes 216 species. The map of collecting localities is presented. Key words: Lepidoptera, Noctuidae, Erebidae, Asia, Kazakhstan, Saur, fauna. INTRODUCTION The fauna of noctuoid moths (the families Erebidae and Noctuidae) of Kazakhstan is still poorly studied. Only the fauna of West Kazakhstan has been studied satisfactorily (Gorbunov 2011). On the faunas of other parts of the country, only fragmentary data are published (Lederer, 1853; 1855; Aibasov & Zhdanko 1982; Hacker & Peks 1990; Lehmann et al. 1998; Benedek & Bálint 2009; 2013; Korb 2013). In contrast to the West Kazakhstan, the fauna of noctuid moths of East Kazakhstan was studied inadequately. -

The Apameini of Israel (Lepidoptera: Noctuidae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Kravchenko, V. D.; Fibiger, M.; Mooser, J.; Junnila, A.; Müller, G. C. The Apameini of Israel (Lepidoptera: Noctuidae) SHILAP Revista de Lepidopterología, vol. 36, núm. 142, junio, 2008, pp. 253-259 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512540015 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Nº 142 9/6/08 11:52 Página 253 SHILAP Revta. lepid., 36 (142), junio 2008: 253-259 CODEN: SRLPEF ISSN:0300-5267 The Apameini of Israel (Lepidoptera: Noctuidae) V. D. Kravchenko, M. Fibiger, J. Mooser, A. Junnila & G. C. Müller Abstract In Israel, 20 species of tribe Apameini belonging to 10 genera have been found to date. Four species are endemic of the Levant (Sesamia ilonae, Luperina kravchenkoi, Gortyna gyulaii and Lenisa wiltshirei). Others are mostly Palaearctic, Mediterranean, Iranian and Irano-Turanian elements. Grassland species of the Apameini are mainly associated with the Temperate region and are univoltine with highest rate of occurrence in May, or in autumn. Most wetland and oases species are multivoltine and occur in oases and riverbeds over the country, though few of the species are extremely localized. The S. ilonae is presently known only from northern area of the Sea of Galilee, while A. deserticola is from oases of the Dead Sea area. -

Nota Lepidopterologica, 25.04.2012, ISSN 0342-7536 ©Societas Europaea Lepidopterologica; Download Unter Und
©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Nota lepi. 35(1): 33-50 33 Additions to the checklist of Bombycoidea and Noctuoidea of the Volgo-Ural region. Part II. (Lepidoptera: Lasiocampidae, Erebidae, Nolidae, Noctuidae) Kari Nupponen ' & Michael Fibiger"^ Merenneidontie 19 D, FI-02320 Espoo, Finland; [email protected] ^ Deceased. 1 1 Received May 20 1 1 ; reviews returned September 20 1 ; accepted 3 December 2011. Subject Editor: Lauri Kaila. Abstract. Faunistic records additional to the recently published lists of Bombycoidea and Noctuoidea of the South Ural Mountains (Nupponen & Fibiger 2002, 2006) are presented, as well as some interesting records from the North Urals and the Lower Volga region. The material in the southern Urals was collected during 2006-2010 in six different expeditions, in North Ural in 2003 and 2007, and in the Lower Volga region in 2001, 2002, 2005, and 2006 in four expeditions. Four species are reported for the first time from Europe: Dichagyris latipennis (Piingeler, 1909), Pseudohermonassa melancholica (Lederer, 1853), Spae- lotis deplorata (Staudinger, 1897), and Xestia albonigra (Kononenko, 1981). Fourteen species are reported for the first time from the southern Urals. Altogether, records of 68 species are reported, including a few corrections to the previous articles. Further illustrations and notes on some poorly known taxa are given. Introduction The fauna of Bombycoidea and Noctuoidea of the southern Ural Mountains has been studied intensely since 1996, and the results of the research during 1996-2005 were published by Nupponen & Fibiger (2002, 2006). Since 2005, several further expedi- tions were made to the Urals by the first author. -

Viruses 2015, 7, 306-319; Doi:10.3390/V7010306 OPEN ACCESS
Viruses 2015, 7, 306-319; doi:10.3390/v7010306 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review History and Current Status of Development and Use of Viral Insecticides in China Xiulian Sun Key Laboratory of Agricultural and Environmental Microbiology, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan 430071, China; E-Mail: [email protected]; Tel.: +86-27-8719-8641; Fax: +86-27-8719-8072 Academic Editors: John Burand and Madoka Nakai Received: 1 December 2014 / Accepted: 14 January 2015 / Published: 20 January 2015 Abstract: The use of insect viruses as biological control agents started in the early 1960s in China. To date, more than 32 viruses have been used to control insect pests in agriculture, forestry, pastures, and domestic gardens in China. In 2014, 57 products from 11 viruses were authorized as commercial viral insecticides by the Ministry of Agriculture of China. Approximately 1600 tons of viral insecticidal formulations have been produced annually in recent years, accounting for about 0.2% of the total insecticide output of China. The development and use of Helicoverpa armigera nucleopolyhedrovirus, Mamestra brassicae nucleopolyhedrovirus, Spodoptera litura nucleopolyhedrovirus, and Periplaneta fuliginosa densovirus are discussed as case studies. Additionally, some baculoviruses have been genetically modified to improve their killing rate, infectivity, and ultraviolet resistance. In this context, the biosafety assessment of a genetically modified Helicoverpa armigera nucleopolyhedrovirus is discussed. Keywords: viral insecticides; commercialization; genetic modification 1. Introduction Research on insect viruses in China started with the Bombyx mori nucleopolyhedrovirus in the mid-1950s [1] and, to date, more than 200 insect virus isolates have been recorded in China. -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties. -

Indiana County Endangered, Threatened and Rare Species List 03/09/2020 County: Elkhart
Page 1 of 4 Indiana County Endangered, Threatened and Rare Species List 03/09/2020 County: Elkhart Species Name Common Name FED STATE GRANK SRANK Insect: Plecoptera (Stoneflies) Acroneuria lycorias Boreal Stonefly SE G5 S1 Perlesta golconda Two-lined Stone SE G2G3 S1 Pteronarcys dorsata American Salmonfly SE G5 S1 Mollusk: Bivalvia (Mussels) Venustaconcha ellipsiformis Ellipse G4 S2 Mollusk: Gastropoda Campeloma decisum Pointed Campeloma SSC G5 S2 Insect: Coleoptera (Beetles) Nicrophorus americanus American Burying Beetle LE SX G3 SX Insect: Hymenoptera Formica ulkei G5 S1 Insect: Lepidoptera (Butterflies & Moths) Apamea lignicolora The Wood-colored Apamea ST G5 S1S2 Apamea nigrior Black-dashed Apamea SR G5 S2S3 Capis curvata Curved Halter Moth ST G5 S2S3 Catocala praeclara Praeclara Underwing SR G5 S2S3 Crambus girardellus Orange-striped Sedge Moth SR GNR S2S3 Dasychira cinnamomea Cinnamon Tussock Moth SE G4 S1 Exyra fax Pitcher Window Moth SE G4 S1S2 Iodopepla u-album White-eyed Borer Moth SR G5 S2 Leucania multilinea Many-lined Wainscot SR G5 S1S2 Macrochilo absorptalis Slant-lined Owlet SR G4G5 S2S3 Macrochilo hypocritalis Twin-dotted Macrochilo SR G4 S2 Melanomma auricinctaria Huckleberry Eye-spot Moth SR G4 S2S3 Papaipema appassionata The Pitcher Plant Borer Moth SE G4 S1 Papaipema speciosissima The Royal Fern Borer Moth ST G4 S2S3 Insect: Odonata (Dragonflies & Damselflies) Sympetrum semicinctum Band-winged Meadowhawk SR G5 S2S3 Insect: Tricoptera (Caddisflies) Setodes oligius A Caddisfly SE G5 S1 Fish Coregonus artedi Cisco SSC -
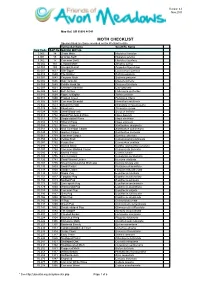
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Community Wind Collaborative – Town of Wellfleet
Massachusetts Technology Collaborative Community Wind Collaborative – Town of Wellfleet SITE FEASIBILITY STUDY B&V Project Number 135720.1200 Funded by the Community Wind Collaborative of the Renewable Energy Trust August 2008 Black & Veatch Corporation 230 Congress Street Suite 802 Boston, MA 02110 Tel: (617) 451-6900 www.bv.com Principal Investigators: Ryan Jacobson, Project Manager Steve Block, Renewable Energy Specialist Justin Ray, Wind Energy Specialist Jason Fields, Wind Energy Specialist Sean Tilley, Wind Energy Specialist © Copyright, Black & Veatch Corporation, 2008. All rights reserved. The Black & Veatch name and logo are registered trademarks of Black & Veatch Holding Company Massachusetts Technology Collaborative Community Wind Collaborative – Town of Wellfleet Table of Contents Table of Contents 1.0 Executive Summary................................................................................................... 1-1 1.1 Study Results ....................................................................................................... 1-1 1.2 List of Recommendations .................................................................................... 1-2 2.0 Introduction................................................................................................................ 2-1 2.1 Background.......................................................................................................... 2-1 2.2 Objective.............................................................................................................