Mammalia, Afrotheria)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MARINE MAMMALS, EXTINCTIONS of Glenn R
MARINE MAMMALS, EXTINCTIONS OF Glenn R. VanBlaricom,* Leah R. Gerber,† and Robert L. Brownell, Jr.‡ *U.S. Geological Survey and University of Washington, †University of California, Santa Barbara, and ‡National Marine Fisheries Service I. Introduction principal source for taxonomic nomenclature, includ- II. Patterns and Case Studies of Extinction in ing common names, is the recent review of Rice (1998). Marine Mammals The order Cetacea includes whales, dolphins, and III. Discussion porpoises (Table I). The ‘‘pinnipedia’’ is a group of species in three families in the mammalian order Carni- vora (Table I). The pinnipeds include the seals, fur seals, sea lions, and walrus. The term pinnipedia is no I. INTRODUCTION longer recognized formally by marine mammal taxono- mists, but it continues to appear in the systematic ver- A. Taxonomic Definition of nacular as a matter of tradition and convenience. The order Sirenia includes the extant manatees and dugong ‘‘Marine Mammals’’ and the extinct Steller’s sea cow (Table I). The order The marine mammals include one extinct order and Desmostylia is the only recognized order of marine three major extant taxa that were or are fully aquatic, mammals to become entirely extinct. in most cases occurring entirely in the marine habitats Two largely terrestrial families of the order Carnivora of the major ocean basins and associated coastal seas also include species recognized as marine mammals and estuaries. In addition, a few species of largely terres- (Table I). Sea otters and chungungos (family Mustel- trial taxa are currently regarded as marine mammals. idae) live entirely or primarily in marine habitats. Polar We consider 127 recent mammal species in total to bears (family Ursidae) also spend a significant propor- be marine mammals for purposes of this review. -

Download Full Article in PDF Format
A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: Pinnipeds and Cetaceans Robert W. BOESSENECKER Department of Geology, University of Otago, 360 Leith Walk, P.O. Box 56, Dunedin, 9054 (New Zealand) and Department of Earth Sciences, Montana State University 200 Traphagen Hall, Bozeman, MT, 59715 (USA) and University of California Museum of Paleontology 1101 Valley Life Sciences Building, Berkeley, CA, 94720 (USA) [email protected] Boessenecker R. W. 2013. — A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: Pinnipeds and Cetaceans. Geodiversitas 35 (4): 815-940. http://dx.doi.org/g2013n4a5 ABSTRACT e newly discovered Upper Miocene to Upper Pliocene San Gregorio assem- blage of the Purisima Formation in Central California has yielded a diverse collection of 34 marine vertebrate taxa, including eight sharks, two bony fish, three marine birds (described in a previous study), and 21 marine mammals. Pinnipeds include the walrus Dusignathus sp., cf. D. seftoni, the fur seal Cal- lorhinus sp., cf. C. gilmorei, and indeterminate otariid bones. Baleen whales include dwarf mysticetes (Herpetocetus bramblei Whitmore & Barnes, 2008, Herpetocetus sp.), two right whales (cf. Eubalaena sp. 1, cf. Eubalaena sp. 2), at least three balaenopterids (“Balaenoptera” cortesi “var.” portisi Sacco, 1890, cf. Balaenoptera, Balaenopteridae gen. et sp. indet.) and a new species of rorqual (Balaenoptera bertae n. sp.) that exhibits a number of derived features that place it within the genus Balaenoptera. is new species of Balaenoptera is relatively small (estimated 61 cm bizygomatic width) and exhibits a comparatively nar- row vertex, an obliquely (but precipitously) sloping frontal adjacent to vertex, anteriorly directed and short zygomatic processes, and squamosal creases. -

Sirenian Feeding Apparatus: Functional Morphology of Feeding Involving Perioral Bristles and Associated Structures
THE SIRENIAN FEEDING APPARATUS: FUNCTIONAL MORPHOLOGY OF FEEDING INVOLVING PERIORAL BRISTLES AND ASSOCIATED STRUCTURES By CHRISTOPHER DOUGLAS MARSHALL A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNrVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REOUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1997 DEDICATION to us simply as I dedicate this work to the memory of J. Rooker (known "Rooker") and to sirenian conservation. Rooker was a subject involved in the study during the 1993 sampling year at Lowry Park Zoological Gardens. Rooker died during the red tide event in May of 1996; approximately 140 other manatees also died. During his rehabilitation at Lowry Park Zoo, Rooker provided much information regarding the mechanism of manatee feeding and use of the perioral bristles. The "mortality incident" involving the red tide event in southwest Florida during the summer of 1996 should serve as a reminder that the Florida manatee population and the status of all sirenians is precarious. Although some estimates suggest that the Florida manatee population may be stable, annual mortality numbers as well as habitat degradation continue to increase. Sirenian conservation and research efforts must continue. ii ACKNOWLEDGMENTS Research involving Florida manatees required that I work with several different government agencies and private parks. The staff of the Sirenia Project, U.S. Geological Service, Biological Resources Division - Florida Caribbean Science Center has been most helpful in conducting the behavioral aspect of this research and allowed this work to occur under their permit (U.S. Fish and Wildlife Permit number PRT-791721). Numerous conversations regarding manatee biology with Dr. -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

How to Cite Complete Issue More Information About This Article
Boletín de la Sociedad Geológica Mexicana ISSN: 1405-3322 Sociedad Geológica Mexicana, A.C. Hernández Cisneros, Atzcalli Ehécatl; González Barba, Gerardo; Fordyce, Robert Ewan Oligocene cetaceans from Baja California Sur, Mexico Boletín de la Sociedad Geológica Mexicana, vol. 69, no. 1, January-April, 2017, pp. 149-173 Sociedad Geológica Mexicana, A.C. Available in: http://www.redalyc.org/articulo.oa?id=94350664007 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Project academic non-profit, developed under the open access initiative Boletín de la Sociedad Geológica Mexicana / 2017 / 149 Oligocene cetaceans from Baja California Sur, Mexico Atzcalli Ehécatl Hernández Cisneros, Gerardo González Barba, Robert Ewan Fordyce ABSTRACT Atzcalli Ehecatl Hernández Cisneros ABSTRACT RESUMEN [email protected] Museo de Historia Natural de la Universidad Autónoma de Baja California Sur, Univer- Baja California Sur has an import- Baja California Sur tiene un importante re- sidad Autónoma de Baja California Sur, ant Cenozoic marine fossil record gistro de fósiles marinos del Cenozoico que Carretera al Sur Km 5.5, Apartado Postal which includes diverse but poorly incluye los restos poco conocidos de cetáceos 19-B, C.P. 23080, La Paz, Baja California Sur, México. known Oligocene cetaceans from del Oligoceno de México. En este estudio Instituto Politécnico Nacional, Centro Inter- Mexico. Here we review the cetacean ofrecemos más detalles sobre estos fósiles de disciplinario de Ciencias Marinas (CICMAR), fossil record including new observa- cetáceos, incluyendo nuevas observaciones Av. Instituto Politécnico Nacional s/n, Col. -

Ore Bin / Oregon Geology Magazine / Journal
THE ORE.-BIN Volume XVI Vol. 16, No.1 THE ORE.-BIN 1 January 1954 Portland, Oregon STATE DEPARTKENT OF GEOLOGY AND KINERAL INDUSTRIES Head Ottice: 1069 State Ottioe Bldg., Portland 1, Oregon Telephone: Columbia 2161, Ext. 488 State Governing Board ~ Kason L. Bingham, Chairman, Portland R. E. Corooran Geologist Niel R. Allen Grants Pass Hollis K. Dole Geologist Austin Dunn Baker L. L. Hoagland Assayer & Chemist Ralph S. Kason Kining Engineer F. W. Libbey, Direotor T. C. Katthews Speotrosoopist Lenin Ramp Geologist K. L. Steere Geologist R. E. stewart Geologist F18ld Ottices 20" First Street, Baker 2'9 S.E. "H" street, Grants Pass N. S. Wagper, Field Geologist David J. White, Field Geologist ****************************** THE ASTORIA LANDSLIDES Erosion to most ot us means the slow, almost imperoeptible, wearing down ot the higher parts ot the earth's crust by running water, wind, or ice. Seemingly mountains remain the same height and stream valleys the same depth throughout the years. The only rapid changes in the landscape which are accepted as normal are the neat exoavations made when new roads, dams, or other man-made structures are constructed. It 1s not sur prising that special attention to the ohoioe of a toundation for an ordinary building is seldom given, tor our experience tells us that the durability ot the structure is infinitely less than that of the ground on whioh it is bull t. It oomes as a shock to us when exceptions to our everyday observations ocour. Suoh is the oase in the land sliding at Astoria, Oregon,at the mouth of the Columbia River. -
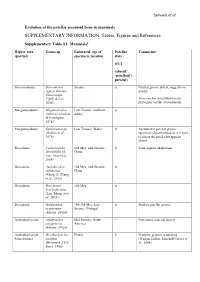
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

25 Squires and Fritsche
25 SQUIRES AND FRITSCHE Isurus sp. TRACE FOSSILS PI. 4, fig. 8 Unidentified burrows Isurus sp. (mako shark) teeth are the most " common shark teeth in the Sespe Creek area. Most The few unidentified burrows collected from the are moderately well preserved, nearly complete or Vaqueros Formation are about 9 cm long and about 1.5 complete, and not rounded by abrasion. Average cm in diameter. Burrows are more abundant in the length of complete specimens is about 1.5 cm; larger Santa Margarita Formation, where the most common type fragments are about 2.5 cm in length. is vertical, 1 to 3 cm in diameter, and straight to slightly curving. Some forms are branching, and at Genus Carcharodon Smith in Muller and Henle, 1838 locality 287, some resemble Ophiomorpha. Carcharodon angustidens L. Agassiz Kingdom PLANTAE PI. 4, fig. 3 Division RHODOPHYCOPHYTA Class RHODOPHYCEAE Carcharodon angustidens L. Agassiz, 1843, p. 255, Order CRYPTQNEMIALES pi. 28, figs. 20-25. Leriche, p. 13-14, pi. 11, figs. 8, 8a, 8b. Family Corallinaceae The figured specimen (PI. 4, fig. 3) of Carchar- Abundant whole and fragmented specimens of. an odon angustidens (great white shark) is a 6-cm-long unidentified coralline alga occur in a thin bed at tooth. Other specimens found include only a few locality 313. Small hemispherical colonies up to fragments. Remains of Carcharodon are previously 2.5 cm in height were collected, but most of the unreported from the Vaqueros Formation in the Sespe specimens are scattered fragments separated by Creek region (Loel and Corey, 1932). medium-grained sandstone. -

IDL-1426.Pdf
The International Development Research Centre is a public corporation created by the Parliament of Canada in 1970 to support research designed to adapt science and technology to the needs of developing countries. The Centre's activity is concentrated in five sectors: agriculture, food and nutrition sciences; health sciences; information sciences; communications; and social sciences. IDRC is financed solely by the Government of Canada; its policies, however, are set by an international Board of Governors. The Centre's headquarters are in Ottawa, Canada. Regional offices are located in Africa, Asia, Latin America, and the Middle East. ©1978 International Development Research Centre Postal Address: Box 8500, Ottawa, Canada KlG 3H9 Head Office: 60 Queen Street, Ottawa Ronald, K. Selley, L.J. Amoroso, E.C. IDRC IDRC-TS13e Biological synopsis of the manatee. Ottawa, IDRC, 1978. 112 p. /IDRC publication/. Monograph on the manatee, an aquatic mammal (/animal species/) of the /tropical zone/s of I Africa south of Sahara/ and /Latin America/, with an extensive /bibliography/ - discusses the /classification/ of the species and subspecies of the genus Trichechus; /morphology/, /physiology/, /behaviour/, /reproduction/, /animal ecology/ (role in /aquatic plant/ /weed control/), and measures for /animal protection/ of the manatee. UDC: 599.55 ISBN: 0-88936-168-1 Microfiche edition available IDRC-TS13e Biological Synopsis of the Manatee K. Ronald, L.J. Selley, and E.C. Amoroso1 College ofBiological Science, University of Guelph, Guelph, Ontario 1 Present address: Agricultural Research Council, Institute of Animal Physiology, Babraham, Cambridge, England. This work was carried out with the aid of a grant from the International Development Research Centre. -

Novitates PUBLISHED by the AMERICAN MUSEUM of NATURAL HISTORY CENTRAL PARK WEST at 79TH STREET, NEW YORK, N.Y
AMERICANt MUSEUM Novitates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 2870, pp. 1-8, figs. 1-3 April 6, 1987 Selected Features of the Desmostylian Skeleton and Their Phylogenetic Implications MICHAEL J. NOVACEK1 AND ANDRE R. WYSS2 ABSTRACT According to several standard descriptions, des- pertaining to these traits are either in error, or have mostylians lack certain specializations shared by alternative phylogenetic implications. Hence, proboscideans, sirenians, and hyracoids. These comparisons of these conditions do not exclude specializations are amastoidy and the serial ar- desmostylians from the superordinal group Teth- rangement ofthe carpals with the concomitant loss ytheria (proboscideans and sirenians) or the more of contact between the lunar and unciform. We inclusive Paenungulata (tethytheres and hyra- argue that original descriptions of desmostylians coids). INTRODUCTION The Desmostylia are an extinct order of be more conservative than sirenians (and, by mammals with specializations ofthe skeleton implication, proboscideans) with respect to suitable for an amphibious mode of life. Al- the reduction ofmastoid exposure (Hay, 1915; though this group was formerly associated Abel, 1922; VanderHoof, 1937) and the se- with the aquatic Sirenia (Simpson, 1945), and rial arrangement ofthe carpal elements (Shi- is now placed within a superordinal category kama, 1966). These differences have influ- Tethytheria, which also includes the Probos- enced more recent students of the problem cidea and Sirenia (McKenna, 1975), these (e.g., Tassy, 1981), who have opted for a close avowed relationships present some prob- association ofProboscidea (including the late lems. According to standard descriptions, Eocene-early Oligocene genus Moeritheri- desmostylians lack certain skeletal special- um) and Sirenia to the exclusion of Desmo- izations shared by Proboscidea and Sirenia. -

Two New Oligocene Desmostylians and a Discussion of Tethytherian Systematics
SMITHSONIAN CONTRIBUTIONS TO PALEOBIOLOGY • NUMBER 59 Two New Oligocene Desmostylians and a Discussion of Tethytherian Systematics Daryl P. Domning, Clayton E. Ray, and Malcolm C. McKenna SMITHSONIAN INSTITUTION PRESS City of Washington 1986 ABSTRACT Domning, Daryl P., Clayton E. Ray, and Malcolm C. McKenna. Two New Oligocene Desmostylians and a Discussion of Tethytherian Systematics. Smith sonian Contributions to Paleobiology, number 59, 56 pages, 23 figures, 1986.— A new genus, comprising two new species of desmostylians, is de scribed from marine Oligocene deposits of the Pacific Northwest. Behemotops proteus, new genus, new species, is based on an immature mandibular ramus and apparently associated skeletal fragments from the middle or (more likely) upper Oligocene lower part of the Pysht Formation of Clallam County, Washington. A related new species, Behemotops emlongi, is founded on a mandibular ramus of an old individual and a mandibular fragment with canine tusk from the uppermost Oligocene (early Arikareean equivalent) Yaquina Formation of Lincoln County, Oregon. The two new species are the most primitive known desmostylians and compare favorably with the primitive Eocene proboscideans Anthracobune and Moeritherium, and to the still more primitive tethythere Minchenella from the Paleocene of China. For many years the Desmostylia were widely regarded as members of the mammalian order Sirenia before being accepted as a taxon coordinate with the Sirenia and Proboscidea (Reinhart, 1953). On the basis of cladistic analysis we go a step further and regard the Desmostylia as more closely related to Proboscidea than to Sirenia because the Desmostylia and Proboscidea are interpreted herein to share a more recent common ancestor than either order does with the Sirenia. -

Addis Ababa University College of Natural Science School of Graduate Studies Department of Zoology
ADDIS ABABA UNIVERSITY COLLEGE OF NATURAL SCIENCE SCHOOL OF GRADUATE STUDIES DEPARTMENT OF ZOOLOGY The Tree Hyrax (Dendrohyrax arboreus): Feeding Behaviour, Activity Patterns and Traditional Medicinal Use in Kafa Zone, Southwest Ethiopia By: Asrat Aero Mamo In Partial Fulfillment of the Requirements for the Degree of Master of Science in Zoological Sciences (Ecological and Systematic Zoology) Advisor: Professor M. Balakrishnan December, 2016 Addis Ababa, Ethiopia ADDIS ABABA UNIVERSITY GRADUATE PROGRAM The Tree Hyrax (Dendrohyrax arboreus): Feeding Behaviour, Activity Patterns and Traditional Medicinal Use in Kafa Zone, Southwest Ethiopia By Asrat Aero Mamo A Thesis Submitted to the School of Graduate Studies of Addis Ababa University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Zoological Science (Ecological and Systematic Zoology) Approved by the Examining Board 1. Prof. M. Balakrishnan (Advisor) _________ |_____ |_____| _____| 2. Dr. Tilaye Wube (Examiner) _________ |_____|_____|______| 3. Dr. Habte Jebessa (Examiner) __________ |_____|_____|______| 4. Prof. Abebe Getahun (Chairperson) _________ |_____ |_____|______| ABSTRACT The Tree Hyrax (Dendrohyrax arboreus): Feeding Behaviour, Activity Patterns and Traditional Medicinal Use in Kafa Zone, Southwest Ethiopia Asrat Aero Mamo Addis Ababa University, 2016 Feeding behaviour, activity pattern and traditional medicinal use of the tree hyrax (Dendrohyrax arboreus) were investigated by direct observations and by questionnaire interview method between July – December 2015 in the Kafa Zone, Southwest Ethiopia. Transect method was used to observe feeding behavior and activity patterns and questionnaire interview was used to determine traditional medicinal use of tree hyrax. Tree hyrax shelters and trees with cavities were located. Activities of hyraxes were observed in the morning, midday and afternoon hours.