Foraging Behavior of the White Ibis.-The Foraging Behavior of Many Ciconiiforms Is Fairly Well Known
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

156 Glossy Ibis
Text and images extracted from Marchant, S. & Higgins, P.J. (co-ordinating editors) 1990. Handbook of Australian, New Zealand & Antarctic Birds. Volume 1, Ratites to ducks; Part B, Australian pelican to ducks. Melbourne, Oxford University Press. Pages 953, 1071-1 078; plate 78. Reproduced with the permission of Bird life Australia and Jeff Davies. 953 Order CICONIIFORMES Medium-sized to huge, long-legged wading birds with well developed hallux or hind toe, and large bill. Variations in shape of bill used for recognition of sub-families. Despite long legs, walk rather than run and escape by flying. Five families of which three (Ardeidae, Ciconiidae, Threskiornithidae) represented in our region; others - Balaenicipitidae (Shoe-billed Stork) and Scopidae (Hammerhead) - monotypic and exclusively Ethiopian. Re lated to Phoenicopteriformes, which sometimes considered as belonging to same order, and, more distantly, to Anseriformes. Behavioural similarities suggest affinities also to Pelecaniformes (van Tets 1965; Meyerriecks 1966), but close relationship not supported by studies of egg-white proteins (Sibley & Ahlquist 1972). Suggested also, mainly on osteological and other anatomical characters, that Ardeidae should be placed in separate order from Ciconiidae and that Cathartidae (New World vultures) should be placed in same order as latter (Ligon 1967). REFERENCES Ligon, J.D. 1967. Occas. Pap. Mus. Zool. Univ. Mich. 651. Sibley, C. G., & J.E. Ahlquist. 1972. Bull. Peabody Mus. nat. Meyerriecks, A.J. 1966. Auk 83: 683-4. Hist. 39. van Tets, G.F. 1965. AOU orn. Monogr. 2. 1071 Family PLATALEIDAE ibises, spoonbills Medium-sized to large wading and terrestial birds. About 30 species in about 15 genera, divided into two sub families: ibises (Threskiornithinae) and spoonbills (Plataleinae); five species in three genera breeding in our region. -

IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2
IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2 Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group Djerba Island, Tunisia, 14th - 18th November 2018 Editors: Jocelyn Champagnon, Jelena Kralj, Luis Santiago Cano Alonso and K. S. Gopi Sundar Editors-in-Chief, Special Publications, IUCN-SSC Stork, Ibis and Spoonbill Specialist Group K.S. Gopi Sundar, Co-chair IUCN Stork, Ibis and Spoonbill Specialist Group Luis Santiago Cano Alonso, Co-chair IUCN Stork, Ibis and Spoonbill Specialist Group Invited Editors for this issue Jocelyn Champagnon, Tour du Valat, Research Institute for the Conservation of Mediterranean Wetlands, Arles, France Jelena Kralj, Institute of Ornithology, Zagreb, Croatia Expert Review Board Hichem Azafzaf, Association “les Amis des Oiseaux » (AAO/BirdLife Tunisia), Tunisia Petra de Goeij, Royal NIOZ, the Netherlands Csaba Pigniczki, Kiskunság National Park Directorate, Hungary Suggested citation of this publication: Champagnon J., Kralj J., Cano Alonso, L. S. & Sundar, K. S. G. (ed.) 2019. Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group. IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2. Arles, France. ISBN 978-2-491451-00-4. Recommended Citation of a chapter: Marion L. 2019. Recent trends of the breeding population of Spoonbill in France 2012- 2018. Pp 19- 23. In: Champagnon J., Kralj J., Cano Alonso, L. S. & Sundar, K. S. G. (ed.) Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group. IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2. Arles, France. INFORMATION AND WRITING DISCLAIMER The information and opinions expressed in this publication belong to the authors. -

Merritt Island National Wildlife Refuge BIRD LIST
Merrritt Island National Wildlife Refuge U.S. Fish & Wildlife Service P.O. Box 2683 Titusville, FL 32781 http://www.fws.gov/refuge/Merritt_Island 321/861 0669 Visitor Center Merritt Island U.S. Fish & Wildlife Service 1 800/344 WILD National Wildlife Refuge March 2019 Bird List photo: James Lyon Merritt Island National Wildlife Refuge, located just Seasonal Occurrences east of Titusville, shares a common boundary with the SP - Spring - March, April, May John F. Kennedy Space Center. Its coastal location, SU - Summer - June, July, August tropic-like climate, and wide variety of habitat types FA - Fall - September, October, November contribute to Merritt Island’s diverse bird population. WN - Winter - December, January, February The Florida Ornithological Society Records Committee lists 521 species of birds statewide. To date, 359 You may see some species outside the seasons indicated species have been identified on the refuge. on this checklist. This phenomenon is quite common for many birds. However, the checklist is designed to Of special interest are breeding populations of Bald indicate the general trend of migration and seasonal Eagles, Brown Pelicans, Roseate Spoonbills, Reddish abundance for each species and, therefore, does not Egrets, and Mottled Ducks. Spectacular migrations account for unusual occurrences. of passerine birds, especially warblers, occur during spring and fall. In winter tens of thousands of Abundance Designation waterfowl may be seen. Eight species of herons and C – Common - These birds are present in large egrets are commonly observed year-round. numbers, are widespread, and should be seen if you look in the correct habitat. Tips on Birding A good field guide and binoculars provide the basic U – Uncommon - These birds are present, but because tools useful in the observation and identification of of their low numbers, behavior, habitat, or distribution, birds. -

Winter Bird Feeding
BirdNotes 1 Winter Bird Feeding birds at feeders in winter If you feed birds, you’re in good company. Birding is one of North America’s favorite pastimes. A 2006 report from the U.S. Fish and Wildlife Service estimates that about 55.5 mil- lion Americans provide food for wild birds. Chickadees Titmice Cardinals Sparrows Wood- Orioles Pigeons Nuthatches Finches Grosbeaks Blackbirds Jays peckers Tanagers Doves Sunflower ◆ ◆ ◆ ◆ ◆ ◆ ◆ Safflower ◆ ◆ ◆ Corn ◆ ◆ ◆ Millet ◆ ◆ ◆ Milo ◆ ◆ Nyjer ◆ Suet ◆ ◆ ◆ ◆ ◆ Preferred ◆ Readily Eaten Wintertime—and the Living’s counting birds at their feeders during selecting the best foods daunting. To Not Easy this winterlong survey. Great Back- attract a diversity of birds, provide a yard Bird Count participants provide variety of food types. But that doesn’t n much of North America, winter valuable data with a much shorter mean you need to purchase one of ev- Iis a difficult time for birds. Days time commitment—as little as fifteen erything on the shelf. are often windy and cold; nights are minutes in mid-February! long and even colder. Lush vegeta- Which Seed Types tion has withered or been consumed, Types of Bird Food Should I Provide? and most insects have died or become uring spring and summer, most dormant. Finding food can be espe- lack-oil sunflower seeds attract songbirds eat insects and spi- cially challenging for birds after a D Bthe greatest number of species. ders, which are highly nutritious, heavy snowfall. These seeds have a high meat-to- abundant, and for the most part, eas- shell ratio, they are nutritious and Setting up a backyard feeder makes ily captured. -
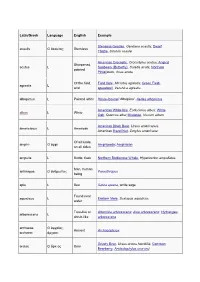
Dwarf Thistle, Cirsi
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

Houde2009chap64.Pdf
Cranes, rails, and allies (Gruiformes) Peter Houde of these features are subject to allometric scaling. Cranes Department of Biology, New Mexico State University, Box 30001 are exceptional migrators. While most rails are generally MSC 3AF, Las Cruces, NM 88003-8001, USA ([email protected]) more sedentary, they are nevertheless good dispersers. Many have secondarily evolved P ightlessness aJ er col- onizing remote oceanic islands. Other members of the Abstract Grues are nonmigratory. 7 ey include the A nfoots and The cranes, rails, and allies (Order Gruiformes) form a mor- sungrebe (Heliornithidae), with three species in as many phologically eclectic group of bird families typifi ed by poor genera that are distributed pantropically and disjunctly. species diversity and disjunct distributions. Molecular data Finfoots are foot-propelled swimmers of rivers and lakes. indicate that Gruiformes is not a natural group, but that it 7 eir toes, like those of coots, are lobate rather than pal- includes a evolutionary clade of six “core gruiform” fam- mate. Adzebills (Aptornithidae) include two recently ilies (Suborder Grues) and a separate pair of closely related extinct species of P ightless, turkey-sized, rail-like birds families (Suborder Eurypygae). The basal split of Grues into from New Zealand. Other extant Grues resemble small rail-like and crane-like lineages (Ralloidea and Gruoidea, cranes or are morphologically intermediate between respectively) occurred sometime near the Mesozoic– cranes and rails, and are exclusively neotropical. 7 ey Cenozoic boundary (66 million years ago, Ma), possibly on include three species in one genus of forest-dwelling the southern continents. Interfamilial diversifi cation within trumpeters (Psophiidae) and the monotypic Limpkin each of the ralloids, gruoids, and Eurypygae occurred within (Aramidae) of both forested and open wetlands. -

At the Dallas World Aquarium Silver-Beaked, Blue-Gray, and Palm Tanagers Can Be Heard More Readily by Shelly Nice, Dallas, TX Than Seen in the Rainforest
ignored with live palm trees being pre Birds ferred. This is probably due to all of the live vegetation. at the Dallas World Aquarium Silver-beaked, Blue-gray, and Palm Tanagers can be heard more readily by Shelly Nice, Dallas, TX than seen in the rainforest. Although he Dallas World Aquarium, the other birds. When food is first intro once you hear then1 they are easily no longer just a place to see duced, it is the smaller birds like the seen. They eat a variety of fruits, fish, is celebrating the first Black -necked and the Green Aracari worms, and seeds. They will stay T that eat first - before the larger toucans together or close by each other. anniversary of a pennanent South Alnerican rainforest exhibit. The pri fly in to eat. Even larger birds such as Although they are the last ones to eat vately owned aquarium allows visitors a curassows will wait for the toucans to fruit, they will be the first ones to arrive glimpse of the flora and fauna from eat before getting their share. when food is put out in the morning. places that many may never see in per , Nesting is another story. The nesting They will perch with toucans just a foot son, such as Lord Howe Island, Banggai sites of the Black-necked Aracari are away and wait their tum. Periodically I land, and Venezuela. The aquarium taken over by the Keel-billed, regard one of these birds will disappear, but it contains several large exhibits of marine less of location. -

Merritt Island National Wildlife Refuge
Merritt Island National Wildlife Refuge Comprehensive Conservation Plan U.S. Department of the Interior Fish and Wildlife Service Southeast Region August 2008 COMPREHENSIVE CONSERVATION PLAN MERRITT ISLAND NATIONAL WILDLIFE REFUGE Brevard and Volusia Counties, Florida U.S. Department of the Interior Fish and Wildlife Service Southeast Region Atlanta, Georgia August 2008 TABLE OF CONTENTS COMPREHENSIVE CONSERVATION PLAN EXECUTIVE SUMMARY ....................................................................................................................... 1 I. BACKGROUND ................................................................................................................................. 3 Introduction ................................................................................................................................... 3 Purpose and Need for the Plan .................................................................................................... 3 U.S. Fish And Wildlife Service ...................................................................................................... 4 National Wildlife Refuge System .................................................................................................. 4 Legal Policy Context ..................................................................................................................... 5 National Conservation Plans and Initiatives .................................................................................6 Relationship to State Partners ..................................................................................................... -

Feed Wild Birds, EC 1554
The Wildlife Garden EC 1554 Reprinted May 2003 $1.50 Feed Wild Birds E. Henning and N. Allen Feeding wild birds has become one of America’s favorite hobbies. It’s easy to attract birds to your yard, and there are many different ways to do so. The most common way is to put out bird feeders for them. Many wild birds such as chickadees, nuthatches, juncos, finches, and jays are regular visitors to feeders in urban areas. Types of food You can buy many types of wild bird foods. They usually consist of whole and shelled seeds that are packaged as a single type or in a variety of mixtures. Different seeds attract different species of birds (see Table 1, page 2). If you’re just getting started with a bird-feeding project, you might want to experiment to see which birds are in your area. Start by putting out a seed mix in an open place and see which kinds of birds you attract. Observe which seeds are wasted or pushed aside. Once birds have started coming to your yard, it is easier to lure them to separate feeding stations. Eric Henning, student, Avoid seed mixes that contain Department of Fisheries only a small amount of sunflower and Wildlife; and Figure 1. Tube feeder with perch. Nancy Allen, Extension seeds. These mixes can be wasteful Illustration courtesy of Wild Birds wildlife instructor; and messy. Commercial wild Unlimited, Inc. Oregon State University 1 Table 1. Common backyard birds and foods they like. Bird Sunflower seeds White millet Nyjer Peanuts Suet Chickadee X* X X House finch/Purple finch X* X X Sparrows X X* X X X Jays X X X American goldfinch X X X* Dark-eyed junco X X* X X X Spotted towhee X* X X Bushtit X Downy/Hairy woodpecker X X Nuthatches X* X X Mourning dove X X* X Quail X* Crow/Raven X Varied thrush X X * Indicates favorite seed choice birdseed mixes usually contain a lot of milo or White proso millet millet, which most wild birds don’t eat. -

Roseate Spoonbill Breeding in Camden County: a First State Nesting Record for Georgia
vol. 76 • 3 – 4 THE ORIOLE 65 ROSEATE SPOONBILL BREEDING IN CAMDEN COUNTY: A FIRST STATE NESTING RECORD FOR GEORGIA Timothy Keyes One Conservation Way Brunswick, GA 31520 [email protected] Chris Depkin One Conservation Way Brunswick, GA 31520 [email protected] Jessica Aldridge 6222 Charlie Smith Sr. Hwy St. Marys, GA 31558 [email protected] Introduction We report the northernmost breeding record of Roseate Spoonbill (Platalea ajaja) on the Atlantic Coast of the U.S. Nesting activity has been suspected in Georgia for at least 5 years, but was first confirmed in June 2011 at a large wading bird colony in St. Marys, Camden County, Georgia. Prior to this record, the furthest northern breeding record for Roseate Spoonbill was in St. Augustine, St. John’s County, Florida, approximately 100 km to the south. This record for Georgia continues a trend of northward expansion of Roseate Spoonbill post-breeding dispersal and breeding ranges. Prior to the plume-hunting era of the mid to late 1800s, the eastern population of Roseate Spoonbill was more abundant and widespread than it is today (Dumas 2000), breeding across much of south Florida. Direct persecution and collateral disturbance by egret plume hunters led to a significant range contraction between 1850 and the 1890s (Allen 1942), limiting the eastern population of Roseate Spoonbills to a few sites in Florida Bay by the 1940s. A low of 15 nesting pairs were documented in 1936 (Powell et al. 1989). By the late 1960s, Roseate Spoonbills began to expand out of Florida Bay, slowly reclaiming some of the territory they had lost. -

Occurrence of Four Species of Ibis Near Dauphin Island, Albama
OCCURRENCE OF FOUR SPECIES OF IBIS NEAR DAUPHIN ISLAND, ALBAMA Gary R. Gaston There are four species of true Ibis (Family: Threskiornithidae) which occurr along the Alabama coastline: Glossy Ibis, White-faced Ibis, White Ibis, and Scarlet Ibis. All four have been sighted near Dauphin Island during the past year, but apparently only the Glossy Ibis nests in the area. The Wood Ibis is actually a member of the stork family (Ciconiidae), and shoul d not be included with this group. Glossy Ibis (Plegadis falcinellus (Linnaeus» have been observed nesting . at Cat Island, Alabama (near Dauphin Island) for several years. A s tudy of the avifauan of Cat Island was undertaken in 1975 , being concluded in September, 1976. Data from this s tudy show that seven Glossy Ibis nests were identified on the island in 1976, and once hatched all of t he young birds survived to f ledgling status. The nests observed each contained 3 eggs, with one exception: on May 26 a nest was located with a clut ch of 6 eggs. This nest was later abandoned and a second nest constructed within a few feet of the first. Photograph records of both young and adult birds were made. The Whi t e-faced Ibis (Plegadis chi hi (Vieillot», though very similar to the Glossy Ibis, does not share Cat Island as nesting habitat . One adult specimen was photogr aphed on the west end of Dauphin Island in September, 1975, but it was not in breeding plumage. The Alabama coastline is included within the nesting range of these birds, but thus far nesting records are not availabl e. -

Birding World 18 (12): 517-526
18-12.qxd 10-Aug-06 12:30 PM Page 517 Sacred Ibis: a new invasive species in Europe Pierre Yésou and Philippe Clergeau Sacred Ibis Threskiornis aethiopicus is closely occurred in the wild in Europe – eg no fossil related to both Black-headed Ibis T. melano- remains have ever been found in Europe cephalus (from the Indian subcontinent) and (Maurer-Chauviré 1993). Sacred Ibises have, Australian White Ibis T. molucca (which breeds however, escaped from captivity and been seen in Australia, New Guinea and some nearby in the wild in Europe since the 19th century, eg islands) – to the point that they are sometimes in Italy (Andreotti et al. 2001), but this remained treated as one species, T. aethiopicus. However, a rare event until about the 1970s when it they are generally regarded as three distinct became fashionable to breed free-flying groups species forming a superspecies (eg del Hoyo et of ibises in zoological gardens. This led to a al. 1992). regular flow of escapes, which in turn led to the The nominate form of Sacred Ibis (T. a. establishment of breeding pairs in the wild, and aethiopicus) is widespread in sub-Saharan breeding populations have now become estab- Africa, while different subspecies breed on lished in Spain, Italy and France, as well as on Madagascar (T. a. bernieri) and Aldabra (T. a. the Canary Islands. Stray birds have also been abbotti, although this is considered by some as reported in other countries. inseparable from bernieri). The species is This addition to the European avifauna has common to very common within its main African been welcomed by some, due to the tameness range, where its population is considered to be and attractiveness of the birds, as well as the stable at an estimated 200,000 to 450,000 indi- aura surrounding the species, which has been viduals (Delany & Scott 2002).