Erythrina, a Potential Source of Chemicals from the Neotropics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antioxidant Activity and Molecular Docking Study of Erythrina × Neillii Polyphenolics
South African Journal of Botany 121 (2019) 470–477 Contents lists available at ScienceDirect South African Journal of Botany journal homepage: www.elsevier.com/locate/sajb Antioxidant activity and molecular docking study of Erythrina × neillii polyphenolics S.K. Gabr a,R.O.Bakra,⁎,E.S.Mostafaa,A.M.El-Fishawyb,T.S.El-Alfyb a Department of Pharmacognosy, Faculty of Pharmacy, October University for Modern Sciences and Arts, 11787 Giza, Egypt. b Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, 11562 Cairo, Egypt article info abstract Article history: Species of genus Erythrina have a great contribution in folk medicine; various species are utilized as a tranquilizer, Received 24 August 2018 to treat insomnia, inflammation and colic. Besides, Erythrina species have reported antioxidant, hepatoprotective Received in revised form 9 December 2018 and anxiolytic activities. Erythrina × neillii is a hybrid obtained through a cross between E. herbacea L. and Accepted 18 December 2018 E. humeana Spreng. It has not been well-studied for its chemical or biological profile; therefore it represents an Available online xxxx interesting field of study. In this study, seven phenolic compounds; two hydrolysable tannins (1,3), one phenolic fl – fi Edited by J Grúz acid (2) and four known avonoids (4 7) were isolated and characterized for the rst time in E × neillii and Erythrina genus except for vitexin (7). Isolated compounds were assessed for their antioxidant activities using Keywords: ORAC assay. 2″-O-galloyl orientin (6) exhibited the highest activity followed by 2″-O-galloyl vitexin (5). Flexible Erythrina × neillii molecular docking on heme oxygenase, an important stress protein that is involved in cellular protection, antiox- Polyphenolics idant and anti-inflammatory activities, justified the antioxidant activity of the isolated compounds. -

Fabaceae Medicinal Flora with Therapeutic Potential in Savanna
Revista Brasileira de Farmacognosia 28 (2018) 738–750 ww w.elsevier.com/locate/bjp Original article Fabaceae medicinal flora with therapeutic potential in Savanna areas in the Chapada do Araripe, Northeastern Brazil a,∗ b a Márcia Jordana Ferreira Macêdo , Daiany Alves Ribeiro , Maria de Oliveira Santos , b a a Delmacia Gonc¸ alves de Macêdo , Julimery Gonc¸ alves Ferreira Macedo , Bianca Vilar de Almeida , a a a,b Manuele Eufrasio Saraiva , Maria Natália Soares de Lacerda , Marta Maria de Almeida Souza a Laboratório de Ecologia Vegetal, Departamento de Ciências Biológicas, Universidade Regional do Cariri, Crato, CE, Brazil b Programa de Pós-graduac¸ ão em Etnobiologia e Conservac¸ ão da Natureza, Universidade Regional do Cariri, Crato, CE, Brazil a b s t r a c t a r t i c l e i n f o Article history: Fabaceae is one of the largest families of ethnopharmacological importance. From this botanical group, Received 11 November 2017 important chemical constituents that act in the treatment and/or healing of various bodily systems arise. Accepted 28 June 2018 The objective of this study was to evaluate the most versatile Fabaceae species and the agreement of use Available online 18 September 2018 among the informants, in the Chapada do Araripe Savanna. The research included five rural communities located in the municipalities of Nova Olinda, Crato, Barbalha, Moreilândia and Exu, covering the states Keywords: of Ceará and Pernambuco. We conducted semi-structured interviews with 126 informants, adopting the Ethnopharmacology snowball technique and using a standardized form. The relative importance and the Informant Consensus Legumes Factor were analyzed for the selection of species with therapeutic potential. -

A Phytochemical and Ethnopharmacological Review of the Genus Erythrina
16 A Phytochemical and Ethnopharmacological Review of the Genus Erythrina João X. de Araújo-Júnior, Mariana S.G. de Oliveira, Pedro G.V. Aquino, Magna S. Alexandre-Moreira and Antônio E.G. Sant’Ana Universidade Federal de Alagoas Brazil 1. Introduction Considered in acient times as a connection to the divine, the use of this medicinal plant is as old as human civilization itself. Whole nations dominated its secrets, often associated with magic and religious rites, searching in nature’s resources to improve life conditions, and increase chances of survival (Herbarium, 2008). In 1978, the World Health Organization (WHO) recognized folk medicine and its beneficial effects to health, during the Alma Ata conference, which published in 1985 that approximatly 80% of the global population, resorted to traditional medicine as their primary health treatment (Herbarium, 2008). Medicinal plants have been used as a means of curing or preventing diseases, now called phytotherapy, in all regions of the world, with regional variations due to the influence of cultural characteristics of the population, as well as its flora, soil and climate (Lewinsohn, 2003). Since the nineteenth century, humanity discovered the endless and diverse therapeutic arsenal present in medicinal plants, due to the discovery of active substances that in their natural state or after chemical transformation showed biological activity, and often already confirmed by popular use and/or proven scientifically (Miguel & Miguel, 2004). According to Yamada (1998) it is necessary to carry out more studies and to propagate medicinal plant utilization as a way to diminish the costs of public health programs since the utilization of these plants may constitute a very useful therapeutic value due their efficacy coupled with low operating costs and the relative ease of obtaining the plants (Matos, 1994). -

PRIMER REPORTE DEL BRUQUIDO EXOTICO Specularius Impressithorax (PIC) (COLEOPTERA: BRUCHIDAE) EN SEMILLAS DE Erythrina Coralloides DC
PRIMER REPORTE DEL BRUQUIDO EXOTICO Specularius impressithorax (PIC) (COLEOPTERA: BRUCHIDAE) EN SEMILLAS DE Erythrina coralloides DC. EN MÉXICO First report of the exotic bruchid Specularius impressithorax (Pic) (Coleoptera: Bruchidae) on seeds of Erythrina coralloides DC. in Mexico. Jesús Romero Nápoles. Instituto de Fitosanidad, Colegio de Postgraduados, México. [email protected]. Palabras Clave: Exótico, Bruchidae, Specularius, Erythrina. Introducción Specularius es un género de la familia Bruchidae que es exclusivo del viejo mundo, éste se caracteriza por la siguiente combinación de caracteres: antena serrada, no sexualmente dimórfica; pronoto campaniforme, con gibosidades e impresiones indistintas y sin carina lateral; estría elitral IV con tubérculo basa; fémur posterior agrandado con un diente ancho en el margen ventral externo y en el margene ventral interno con un diente grande seguido de dos o tres dientes más pequeños; tibia posterior agrandada, carinada y ligeramente arqueada basalmente; genitalia con el lóbulo medio moderadamente largo, valva ventral triangular y saco interno sin escleritos grandes; lóbulos laterales comprimidos y sin modificaciones en la parte apical. El género fue descrito por Bridwell en 1938, para incluir a la especie tipo S. erythrinae; sin embargo, Decelle (1951) puso a la especie bajo sinonimía de S. impressithorax (Pic, 1932). El nombre genérico refiere a un área en el pigidio que es circular, glabra y tiene parecido a un espejo (speculum), ésta se encuentra presente en ambos sexos; sin embargo de las nueve especies registradas sólo está presente en S. impressithorax y S. erythraeus. Las hembras de la mayoría de las especies de Gibbobruchus, género del nuevo mundo, se caracterizan también en parte por tener un speculum; sin embargo, la relación entre Gibbobruchus y Specularius no se ha definido todavía, a pesar de la similitud que presentan ambos géneros. -

Antifeedant Activities of the Erythrinaline Alkaloids from Erythrina Latissima Against Spodoptera Littoralis (Lepidoptera Noctuidae)
ORIGINAL ARTICLE Rec. Nat. Prod . 3:2 (2009) 96-103 Antifeedant Activities of the Erythrinaline Alkaloids from Erythrina latissima against Spodoptera littoralis (Lepidoptera noctuidae) Wanjala W. Cornelius 1*, Teresa Akeng’a 2, George O Obiero 2 and Kweyu P. Lutta 3 1Department of Chemistry, Kigali Institute of Science and Technology, P.O Box 3900, Kigali, RWANDA 2School of Chemical and Minerals Engineering, North West University, P.O Box 558, Potchefstroom 2520, South Africa 3Institute of Tropical Medicine and Infectious Diseases, Jomo Kenyatta University of Agriculture and Technology, P.O Box 62000-00200, Nairobi, KENYA. (Received October 28, 2008; Revised March 03, 2009; Accepted March 5, 2009) Abstract: The antifeedant activities of the Erythrina alkaloids from the seeds, seed pods and flowers of Erythrina latissima were investigated in laboratory dual- choice bioassays using third-instar Spodoptera littoralis (Boisduval) larvae. The new compound (+)-11 β-methoxy-10-oxoerysotramidine ( 1) from the flowers, showed potent dose dependant activity at concentration ≥ 500 ppm while (+)-10,11-dioxoerysotramidine ( 2) also new from the flowers showed potent dose dependant activity at concentration ≥100 ppm. Three known compounds (+)-erysotrine, (+)-erysotramidine, (+)-erythraline, (+)-11 β-hydroxyerysotramidine showed potent dose dependant antifeedant activity at concentrations ≥100 ppm while (+)-10,11-dioxoerysotrine and (+)-11 β- hydroxyerysotramidine also a known compounds showed potent dose dependant antifeedant activity at concentrations ≥ 300 ppm. Three known compounds (+)-11 β-methoxyerysotramidine, (+)-8-oxoerythraline and (+)-15(16) β-D-glucoerysodine showed no appreciable change in antifeedant activity with concentration change. Keywords: Erythrina alkaloids; Erythrina latissima ; antifeedant activity; Spodoptera littoralis. * Corresponding author: E-Mail: c.wanjala@ yahoo.com , Phone: +250547696. -

Erythrina Spp., Fabaceae) in the Ancient Gardens of Naples (Campania, Italy)
plants Article DNA Barcoding to Confirm the Morphological Identification of the Coral Trees (Erythrina spp., Fabaceae) in the Ancient Gardens of Naples (Campania, Italy) Adriana De Luca 1 ID , Giancarlo Sibilio 2,* ID , Paolo De Luca 2 and Emanuele Del Guacchio 2 1 Dipartimento di Medicina Veterinaria e Produzioni Animali, Università degli Studi di Napoli Federico II, Via Delpino 1, 80137 Napoli, Italy; [email protected] 2 Botanical Garden of Naples, Università degli Studi di Napoli Federico II, Via Foria 223, 80139 Napoli, Italy; [email protected] (P.D.L.); [email protected] (E.D.G.) * Correspondence: [email protected] Received: 4 April 2018; Accepted: 4 June 2018; Published: 6 June 2018 Abstract: The coral trees (genus Erythrina) have been fostering great interest among the botanists and gardeners of Naples, since their arrival in Europe in the second half of the 18th century. Numerous species were present in the royal and private botanical gardens of the region, but their number has decreased today. The purpose of this work was to verify which species occur nowadays in the public areas of Naples and associate them with the historical information about their introduction. The identification was carried out also by molecular methods, by means of sequencing nuclear and chloroplast DNA markers. The comparison of the sequences obtained for the specimens present in Naples with those present in the literature, together with a morphological examination, allowed us to identify with accuracy the species anciently introduced or nowadays cultivated in Naples. Keywords: botanical garden; botanical history; Dehnhardt; DNA barcoding; urban gardens 1. -

Medicinal Plant Research Volume 10 Number 39, 17 October, 2016 ISSN 1996-0875
Journal of Medicinal Plant Research Volume 10 Number 39, 17 October, 2016 ISSN 1996-0875 ABOUT JMPR The Journal of Medicinal Plant Research is published weekly (one volume per year) by Academic Journals. The Journal of Medicinal Plants Research (JMPR) is an open access journal that provides rapid publication (weekly) of articles in all areas of Medicinal Plants research, Ethnopharmacology, Fitoterapia, Phytomedicine etc. The Journal welcomes the submission of manuscripts that meet the general criteria of significance and scientific excellence. Papers will be published shortly after acceptance. All articles published in JMPR are peer reviewed. Electronic submission of manuscripts is strongly encouraged, provided that the text, tables, and figures are included in a single Microsoft Word file (preferably in Arial font). Contact Us Editorial Office: [email protected] Help Desk: [email protected] Website: http://www.academicjournals.org/journal/JMPR Submit manuscript online http://ms.academicjournals.me/ Editors Prof. Akah Peter Achunike Prof. Parveen Bansal Editor-in-chief Department of Biochemistry Department of Pharmacology & Toxicology Postgraduate Institute of Medical Education and University of Nigeria, Nsukka Research Nigeria Chandigarh India. Associate Editors Dr. Ravichandran Veerasamy AIMST University Dr. Ugur Cakilcioglu Faculty of Pharmacy, AIMST University, Semeling - Elazıg Directorate of National Education 08100, Turkey. Kedah, Malaysia. Dr. Jianxin Chen Dr. Sayeed Ahmad Information Center, Herbal Medicine Laboratory, Department of Beijing University of Chinese Medicine, Pharmacognosy and Phytochemistry, Beijing, China Faculty of Pharmacy, Jamia Hamdard (Hamdard 100029, University), Hamdard Nagar, New Delhi, 110062, China. India. Dr. Hassan Sher Dr. Cheng Tan Department of Botany and Microbiology, Department of Dermatology, first Affiliated Hospital College of Science, of Nanjing Univeristy of King Saud University, Riyadh Traditional Chinese Medicine. -

SABONET Report No 18
ii Quick Guide This book is divided into two sections: the first part provides descriptions of some common trees and shrubs of Botswana, and the second is the complete checklist. The scientific names of the families, genera, and species are arranged alphabetically. Vernacular names are also arranged alphabetically, starting with Setswana and followed by English. Setswana names are separated by a semi-colon from English names. A glossary at the end of the book defines botanical terms used in the text. Species that are listed in the Red Data List for Botswana are indicated by an ® preceding the name. The letters N, SW, and SE indicate the distribution of the species within Botswana according to the Flora zambesiaca geographical regions. Flora zambesiaca regions used in the checklist. Administrative District FZ geographical region Central District SE & N Chobe District N Ghanzi District SW Kgalagadi District SW Kgatleng District SE Kweneng District SW & SE Ngamiland District N North East District N South East District SE Southern District SW & SE N CHOBE DISTRICT NGAMILAND DISTRICT ZIMBABWE NAMIBIA NORTH EAST DISTRICT CENTRAL DISTRICT GHANZI DISTRICT KWENENG DISTRICT KGATLENG KGALAGADI DISTRICT DISTRICT SOUTHERN SOUTH EAST DISTRICT DISTRICT SOUTH AFRICA 0 Kilometres 400 i ii Trees of Botswana: names and distribution Moffat P. Setshogo & Fanie Venter iii Recommended citation format SETSHOGO, M.P. & VENTER, F. 2003. Trees of Botswana: names and distribution. Southern African Botanical Diversity Network Report No. 18. Pretoria. Produced by University of Botswana Herbarium Private Bag UB00704 Gaborone Tel: (267) 355 2602 Fax: (267) 318 5097 E-mail: [email protected] Published by Southern African Botanical Diversity Network (SABONET), c/o National Botanical Institute, Private Bag X101, 0001 Pretoria and University of Botswana Herbarium, Private Bag UB00704, Gaborone. -

Erythrina Velutina Willd
Revista Verde de Agroecologia e Desenvolvimento Sustentável Revisão Bibliográfica http://revista.gvaa.com.br ISSN 1981-8203 Erythrina velutina Willd. - Fabaceae: Árvore de múltiplos usos no nordeste brasileiro Erythrina velutina Willd. Fabaceae: Tree multiple uses in the brazilian northeast Laércio Wanderley dos Santos1, Maria de Fátima Barbosa Coelho2, Rodrigo Aleixo Brito de Azevedo3, Ana Késya Bernardo Lima4, José Wilson Nascimento de Souza4 Resumo: O mulungu (Erythrina velutina Willd.) é uma árvore que ocorre no nordeste do Brasil e usada como medicinal, madeireira, artesanal, ornamental e como componente de sistema agroflorestais. A partir de consulta a Bases Bibliográficas foi realizada uma revisão sobre a espécie tratando os aspectos taxonômicos e botânicos, composição química e aplicações na fitoterapia, propagação e conservação. Palavras-chave: Erythrina velutina, fitoterapia, propagação Abstract: The coral tree is a tree of different uses in northeastern Brazil. From consultation to Bibliographic Databases is a review on the species and taxonomic aspects dealing with botanists, chemical composition and applications in phytotherapy, propagation and conservation. Keywords: Erythrina velutina, phytotherapy, propagation INTRODUÇÃO produção de mudas por este método, depende de um bom enraizamento do material propagativo. A clonagem de O mulungu (Erythrina velutina Wildenow - mulungu pode ser realizada por meio de estacas Fabaceae) é uma espécie arbórea que ocorre em todo o procedentes de mudas com 06 a 12 meses de idade, nordeste brasileiro e é utilizada na medicina popular com podendo-se obter até 95% de enraizamento (SANTOS & ação comprovada por pesquisas científicas (MAIA, 2004; COELHO, 2011). LORENZI & MATOS 2008). A espécie é utilizada ainda Erythrina velutina Willdenow é uma árvore com fins madeireiros, artesanais, ornamentais e decídua, heliófita, nativa da Caatinga da região semiárida industriais. -

Plant Natural Metabolites As Antimicrobial Agents: a Review
World Research Journal of Antimicrobial Agents ISSN: 2320-3390 & E-ISSN: 2320-5652,Volume 2, Issue 1, 2013, pp. 021-029. Available online at http://www.bioinfopublication.org/jouarchive.php?opt=&jouid=BPJ0000016 PLANT NATURAL METABOLITES AS ANTIMICROBIAL AGENTS: A REVIEW GOEL A.1* AND SHARMA K.2 1Amity Institute of Microbial Biotechnology, Amity University, Noida- 201 301, UP, India. 2Department of Biotechnology, Mohanlal Sukhadia University, Udaipur- 313 001, Rajasthan, India. *Corresponding Author: Email- [email protected] Received: November 07, 2013; Accepted: December 09, 2013 Abstract- In recent years utilization and search of drugs and dietary supplements derived from plants have been accelerated. Scientists and researchers are combing the Earth for phytochemicals and "leads" which could be developed for infectious diseases treatment. Pathogenic microbes have the ability to develop resistance against synthetic formulations. Apart from this, synthetic formulations are very toxic and re- sponsible to destroy the soil fertility and ecological balance. Plant based formulations are least toxic and better for environment balance so it can be replace by synthetic formulations. Secondary metabolites found in plants are the main reason for their antimicrobial potential. Plants are rich in wide variety of secondary metabolites viz. tannins, terpenoids, alkaloids, and flavonoids. This review attempts to summarize the current status of microbiological screening efforts as well as in vivo studies of plant extracts and phytochemicals, their effectiveness and toxici- ty against various microbes. Keywords- Plant Extracts, Antimicrobial Activity, Phytochemicals, Secondary Metabolites Introduction concerned with the advanced state of knowledge on the general Primary healthcare systems involve use of medicinal plants as an principles and details of treatment [7]. -
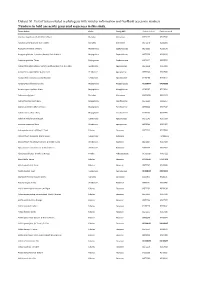
Dataset S1. List of Taxa Included in Phylogeny with Voucher Information and Genbank Accession Numbers
Dataset S1. List of taxa included in phylogeny with voucher information and GenBank accession numbers. Numbers in bold are newly generated sequences in this study. Taxon Author Order Family APG Genbank rbcLa Genbank matK Abutilon angulatum (Guill. & Perr.) Mast. Malvales Malvaceae JX572177 JX517944 Abutilon sonneratianum (Cav.) Sweet Malvales Malvaceae JX572178 JX518201 Acalypha chirindica S.Moore Malpighiales Euphorbiaceae JX572236 JX518178 Acalypha glabrata f. pilosior (Kuntze) Prain & Hutch. Malpighiales Euphorbiaceae JX572238 JX518120 Acalypha glabrata Thunb. Malpighiales Euphorbiaceae JX572237 JX517655 Acokanthera oblongifolia (Hochst.) Benth. & Hook.f. ex B.D.Jacks. Gentianales Apocynaceae JX572239 JX517911 Acokanthera oppositifolia (Lam.) Codd Gentianales Apocynaceae JX572240 JX517680 Acokanthera rotundata (Codd) Kupicha Gentianales Apocynaceae JF265266 JF270623 Acridocarpus chloropterus Oliv. Malpighiales Malpighiaceae KU568077 KX146299 Acridocarpus natalitius A.Juss. Malpighiales Malpighiaceae JF265267 JF270624 Adansonia digitata L. Malvales Malvaceae JQ025018 JQ024933 Adenia fruticosa Burtt Davy Malpighiales Passifloraceae JX572241 JX905957 Adenia gummifera (Harv.) Harms Malpighiales Passifloraceae JX572242 JX517347 Adenia spinosa Burtt Davy Malpighiales Passifloraceae JF265269 JX905950 Adenium multiflorum Klotzsch Gentianales Apocynaceae JX572243 JX517509 Adenium swazicum Stapf Gentianales Apocynaceae JX572244 JX517457 Adenopodia spicata (E.Mey.) C.Presl Fabales Fabaceae JX572245 JX517808 Afrocanthium lactescens (Hiern) Lantz -

Ancient and Current Distributions of Erythrina Crista-Galli L. (Fabaceae) in South America
Floresta e Ambiente 2019; 26(2): e11442017 https://doi.org/10.1590/2179-8087.114417 ISSN 2179-8087 (online) Original Article Conservation of Nature Ancient and Current Distributions of Erythrina crista-galli L. (Fabaceae) in South America Luciano Moura de Mello1 , Rafael Lemos2 , Alcemir Marques2 , Valdir Marcos Stefenon2 1Colégio Militar de Santa Maria, Santa Maria/RS, Brasil 2Universidade Federal do Pampa – UNIPAMPA, São Gabriel/RS, Brasil ABSTRACT Erythrina crista-galli is a native tree from South America with an important role on pharmaceutical studies. The objective of this study was to model the ancient and current ecological niches distribution of E. crista-galli in South America, contributing to the discussion about species management and conservation. A reduction in the potential area of species occurrence was detected by comparing past and present distribution. Based on the obtained results, it is expected that this species tends to expand its frontiers if the natural dynamic of the extant populations is guaranteed in southern South America. Therefore, management and conservation of E. crista-galli should focus on allowing the expansion of native forest formations where this species occurs. Additionally, further studies on the medicinal properties of the species may valorize and promote a higher interest in rational exploitation and conservation of E. crista-galli in its natural environment. Keywords: maximum entropy, corticeira-do-banhado, Brazilian Pampa. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/9 Mello LM, Lemos R, Marques A, Stefenon VM Floresta e Ambiente 2019; 26(2): e11442017 1.