Ecology of Mountain Goats in the Absaroka Range, South-Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Black Hills Hydrology Study —By Janet M
Prepared in cooperation with the South Dakota Department of Environment and Natural Resources and the West Dakota Water Development District The Black Hills Hydrology Study —By Janet M. Carter, Daniel G. Driscoll, and Joyce E. Williamson o Introduction 104o 45' 103 30' Indian Horse o Belle Fourche EXPLANATION 44 45' Reservoir Cr The Black Hills area is an impor- Owl Newell Outcrop of Madison Limestone BELLE Creek Creek tant resource center that provides an Nisland Outcrop of Minnelusa Formation F BELLE FOURCHE OU economic base for western South RCHE RIVER Approximate extent of the Black Hay Creek R E BUTTE CO Vale Hills area, represented by Dakota through tourism, agriculture, I V ER R MEADE CO REDWAT LAWRENCE CO generalized outer extent of the timber, and mineral resources. Water Cox the outcrop of Inyan Kara Saint Creek Lake Crow Onge Group originating from the area is used for Creek reek municipal, industrial, agricultural, and 30' Gulch Spearfish C Whitewood Bear x Gulch Butte Bottom Creek e recreational purposes throughout ls Bear a Creek F Whitewood Butte Higgins much of western South Dakota. The Cr Creek Squ STURGIS Spearfish a Central Tinton Cr w li Iron CityCr ka ood DEADWOOD l o Black Hills area also is an important Cr w A 103 ad 15' Beaver Cr e D Cr Lead Bear h nnie Cr s A berry recharge area for aquifers in the north- i traw f S r Cr Creek Tilford a hitetail e W p Cheyenne Elk S ern Great Plains. Crossing Little Creek Roubaix ek Creek N Elk re Elk Little C Population growth, resource devel- . -

Custer Gallatin National Forest Beartooth Ranger District Information Packet
CUSTER GALLATIN NATIONAL FOREST BEARTOOTH RANGER DISTRICT INFORMATION PACKET www.fs.usda.gov/custergallatin Did You Know? • The highest 41 peaks in Montana are in the Beartooth Mountains. 22 of these are over 12,000ft. • Granite Peak is Montana’s highest peak, at 12,799ft. It is known for its remoteness and extreme weather. • The Absaroka- Beartooth Wilderness is the 6th largest wilderness area in the lower 48 states. • There are over 300 lakes and 10 major sub-alpine tundra plateaus in the Beartooths, with even more lakes across the Absaroka-Beartooth Wilderness. • At 3.96 billion years old, rock samples from the Beartooths are some of the oldest rocks on Earth. • The Beartooth Highway reaches an altitude of 10, 947 ft. and is often considered one of the most beautiful roads in America. 406-446-2103 ∙ 6811 Hwy 212, Red Lodge, MT 59068 You are camping in bear country. Wilderness Restrictions and Regulations The Beartooth Ranger District has an area of 587,000 acres. Of this, 345,000 acres are within the Absaroka-Beartooth Wilderness. The boundary of the Absaroka-Beartooth Wilderness continues west into the Gallatin National Forest (in all, the Absaroka-Beartooth Wilderness is 943,626 acres). General Use 15 people is the maximum group size 16 days at a camp site is the maximum camp stay limit No camping/campfires within 200 feet of a lake No camping/campfires within 100 feet of flowing water No use/possession of motorized vehicles, motorboats, chainsaws and other mechanized equipment Bicycles, wagons, carts, hang gliders or other mechanized equipment cannot be possessed or used Dispose of human waste properly. -

The University of Wyoming Board of Trustees' Report
THE UNIVERSITY OF WYOMING BOARD OF TRUSTEES’ REPORT September 6-8, 2007 The Final Report can be found on the University of Wyoming Board of Trustees website at www.uwyo.edu/trustees/meetings University of Wyoming Mission Statement (April 2002) The University of Wyoming aspires to be one of the nation’s finest public land-grant research universities, dedicated to serving as a statewide resource for accessible and affordable higher education of the highest quality, rigorous scholarship, technology transfer, economic and community development, and responsible stewardship of our cultural, historical, and natural resources. In the exercise of our primary mission to teach and educate students, we seek to provide academic and co-curricular opportunities that will: • Expose students to the frontiers of scholarship and creative activity, and the complexities of an interdependent world; • Ensure individual interactions among students, faculty, and staff; • Nurture an environment that values and manifests diversity, free expression, academic freedom, personal integrity, and mutual respect; and • Promote opportunities for personal growth, physical health, athletic competition, and leadership development for all members of the University community. As Wyoming’s only university, we are committed to outreach and service that extend our human talent and technological capacity to serve the people in our communities, our state, the nation, and the world. The primary vehicles for identifying the specific actions and resource allocations needed to achieve this complex mission are the University’s Academic Plan, Support Services Plan, and Capital Facilities Plan, each revised periodically. TRUSTEES OF THE UNIVERSITY OF WYOMING AGENDA September 6-8, 2007 Thursday, September 6, 2007 9:30 a.m.-11:45 p.m. -

It's Unfair to the People of This Area for Us To
“It’s unfair to the people of this area for us to collect taxes from our customers to help TVA [Tennessee Valley Authority] sell power at a lower price to their customers.” NEIL SIMPSON, President, Black Hills Power and Light Company 60 Expanding Futures on the Great Plains 4 EXPANDING FUTURES ON THE GREAT PLAINS Black Hills Power and Light continued to expand. The company absorbed smaller utilities. It offered power and transmission services to other areas in collaboration with public power agencies and rural electric cooperatives. But tensions with the rural cooperatives were building over territories and customers. As the federal government began to construct dams and hydroelectric facilities on the Missouri River, company officials scrambled to hold onto Black Hills Power and Light’s market and customers. 61 Expanding Futures on the Great Plains Govenor Peter Norbeck’s plan to build a dam dams on the river would revive the state’s proponents of the public power district bill were and hydroelectric facilities on the Missouri River economy. Their efforts to encourage the federal able to convince legislators that new districts after World War I died for lack of sufficient government to build a series of dams gained were needed to secure the power to be generated demand, but the idea lingered in the minds of momentum in 1943 after spring floods caused by Missouri River hydroelectric plants. The public many policymakers in Pierre and Washington, major damage to downstream communities, power district bill passed in 1950. D.C. After drought, depression and war, South especially Omaha, Nebraska. -

Geologic Map of the Red Lodge Area, Carbon
GEOLOGIC MAP OF THE RED LODGE AREA, CARBON COUNTY, MONTANA by David A. Lopez Montana Bureau of Mines and Geology Open-File Report MBMG 524 2005 This map has been reviewed for conformity with technical and editorial standards of the Montana Bureau of Mines and Geology. Partial support has been provided by the STATEMAP component of the National Cooperative Geologic Mapping Program of the U.S. Geological Survey under Contract Number 04HQAG0079. Kalispell MONTANA 15 Great Falls 90 Missoula Helena 94 Butte Billings Bozeman 90 90 15 110° 109° Big Timber YELLOWSTONE CO 94 Y Billings r 90 e r e l SWEET GRASS CO v l owsto e i n v R e Riv i r e 90 R e r ld u ne o to B ws STILLWATER CO lo Columbus el Y 45°30' e 78 n o r t ive 212 s R w r o te l a l w e ll Y ti S e h CARBON CO t BIG HORN CO f o Luther k STUDY r o Red Lodge 72 F AREA s rk la C 310 N PARK CO 212 45° 10 0 10 20 Miles Figure 1. Location map of the study area. 1 2 DESCRIPTION OF MAP UNITS SURFICIAL DEPOSITS af Artificial fill—Mine tailings and fill in the Rock Creek valley in northern part of the town of Red Lodge. Qal Alluvium (Holocene)—Gravel, sand, silt, and clay along active stream channels. Qc Colluvium (Holocene and Pleistocene)—Locally derived slope-wash depositsmainly of sand, silt, and clay. Typically thin veneer concealing bedrock, but locally as thick as 30 ft (9 m). -

A Publication of the Wyoming Native Plant Society
Castilleja A Publication of the Wyoming Native Plant Society October 2004, Volume 23, No. 3 www.uwyo.edu/wyndd/wnps/wnps_home.htm In this issue: Relicts and Refugia . 1 Floristic Diversity of Wyoming Counties . 3 Botanical Novitiates Find Botanical Novelty . 4 Critical Habitat for the Colorado Butterfly Plant . 5 Requiem for a Lawnmower – review. 6 Rocky Mountain Natural History – review . .7 Whitebark Pine - excerpt. 8 Cynoglossum boreale – addition to the state flora 9 Raising Livestock and Lowering Carbon Dioxide . 10 Scholarship Announcement . 11 Natives vs. Imposters. 12 Relicts and Refugia By Bonnie Heidel For all of the breath-taking alpine topography of the Medicine Bow Range, some of its heart-thumping botany lies low across rolling expanses. Three years and three stages of peatland research have documented vast Above: Eriophorum gracile (slender cotton-grass) is montane fen systems in the Medicine Bow circumboreal, with outlying distribution in northwestern Range, refugia for eleven rare Wyoming Wyoming, the Medicine Bow Range and South Park in vascular plant species of concern including five Colorado By B. Heidel relict species previously unknown from southern Wyoming. peatlands harbor close to 10% of the rare Peatland rare species are disjunct or Wyoming plant species of concern. peripheral as they are present in Wyoming, Botanists took a plunge into peatlands denizens of high latitudes, not state and with pilot site surveys on the Medicine Bow and regional endemics that are the focus of most the Shoshone national forests to compile a Wyoming Natural Diversity Database botany working list of peatland rare species, flora, and research. However, review of the Wyoming vegetation at a small number of known or plant species of concern list in 2002 compared inferred peatland study sites (Heidel and against regional peatland floras indicated that Laursen 2003 a, b; Mellmann-Brown 2004). -

Characterization of Ecoregions of Idaho
1 0 . C o l u m b i a P l a t e a u 1 3 . C e n t r a l B a s i n a n d R a n g e Ecoregion 10 is an arid grassland and sagebrush steppe that is surrounded by moister, predominantly forested, mountainous ecoregions. It is Ecoregion 13 is internally-drained and composed of north-trending, fault-block ranges and intervening, drier basins. It is vast and includes parts underlain by thick basalt. In the east, where precipitation is greater, deep loess soils have been extensively cultivated for wheat. of Nevada, Utah, California, and Idaho. In Idaho, sagebrush grassland, saltbush–greasewood, mountain brush, and woodland occur; forests are absent unlike in the cooler, wetter, more rugged Ecoregion 19. Grazing is widespread. Cropland is less common than in Ecoregions 12 and 80. Ecoregions of Idaho The unforested hills and plateaus of the Dissected Loess Uplands ecoregion are cut by the canyons of Ecoregion 10l and are disjunct. 10f Pure grasslands dominate lower elevations. Mountain brush grows on higher, moister sites. Grazing and farming have eliminated The arid Shadscale-Dominated Saline Basins ecoregion is nearly flat, internally-drained, and has light-colored alkaline soils that are Ecoregions denote areas of general similarity in ecosystems and in the type, quality, and America into 15 ecological regions. Level II divides the continent into 52 regions Literature Cited: much of the original plant cover. Nevertheless, Ecoregion 10f is not as suited to farming as Ecoregions 10h and 10j because it has thinner soils. -

Related Magmatism in the Upper Wind River Basin, Wyoming (USA), GEOSPHERE; V
Research Paper THEMED ISSUE: Cenozoic Tectonics, Magmatism, and Stratigraphy of the Snake River Plain–Yellowstone Region and Adjacent Areas GEOSPHERE The leading wisps of Yellowstone: Post–ca. 5 Ma extension- related magmatism in the upper Wind River Basin, Wyoming (USA), GEOSPHERE; v. 14, no. 1 associated with the Yellowstone hotspot tectonic parabola doi:10.1130/GES01553.1 Matthew E. Brueseke1, Anna C. Downey1, Zachary C. Dodd1, William K. Hart2, Dave C. Adams3, and Jeff A. Benowitz4 12 figures; 2 tables; 1 supplemental file 1Department of Geology, Kansas State University, 108 Thompson Hall, Manhattan, Kansas 66506, USA 2Department of Geology and Environmental Earth Science, Miami University, 118C Shideler Hall, Oxford, Ohio 45056, USA 3Box 155, Teton Village, Wyoming 83025, USA CORRESPONDENCE: brueseke@ ksu .edu 4Geophysical Institute and Geochronology Laboratory, University of Alaska Fairbanks, Fairbanks, Alaska 99775, USA CITATION: Brueseke, M.E., Downey, A.C., Dodd, Z.C., Hart, W.K., Adams, D.C., and Benowitz, J.A., 2018, The leading wisps of Yellowstone: Post–ca. 5 Ma ABSTRACT the issue of linking volcanic events to a specific driving mechanism (Fouch, extension-related magmatism in the upper Wind River 2012; Kuehn et al., 2015). Complicating matters, magmatism often continues Basin, Wyoming (USA), associated with the Yellow- The upper Wind River Basin in northwest Wyoming (USA) is located ~80– long after (e.g., millions of years) the upper plate has been translated away stone hotspot tectonic parabola: Geosphere, v. 14, no. 1, p. 74–94, doi:10.1130/GES01553.1. 100 km southeast of the Yellowstone Plateau volcanic field. While the upper from an upwelling plume (Bercovici and Mahoney, 1994; Sleep, 2003; Shervais Wind River Basin is a manifestation of primarily Cretaceous to Eocene Lara- and Hanan, 2008; Jean et al., 2014). -

Yellowstone National Park Geologic Resource Evaluation Scoping
Geologic Resource Evaluation Scoping Summary Yellowstone National Park This document summarizes the results of a geologic resource evaluation scoping session that was held at Yellowstone National Park on May 16–17, 2005. The NPS Geologic Resources Division (GRD) organized this scoping session in order to view and discuss the park’s geologic resources, address the status of geologic maps and digitizing, and assess resource management issues and needs. In addition to GRD staff, participants included park staff and cooperators from the U.S. Geological Survey and Colorado State University (table 1). Table 1. Participants of Yellowstone’s GRE Scoping Session Name Affiliation Phone E-Mail Bob Volcanologist, USGS–Menlo Park 650-329-5201 [email protected] Christiansen Geologist/GRE Program GIS Lead, NPS Tim Connors 303-969-2093 [email protected] Geologic Resources Division Data Stewardship Coordinator, Greater Rob Daley 406-994-4124 [email protected] Yellowstone Network Supervisory Geologist, Yellowstone Hank Heasler 307-344-2441 [email protected] National Park Geologist, NPS Geologic Resources Bruce Heise 303-969-2017 [email protected] Division Cheryl Geologist, Yellowstone National Park 307-344-2208 [email protected] Jaworowski Katie Geologist/Senior Research Associate, 970-586-7243 [email protected] KellerLynn Colorado State University Branch Chief, NPS Geologic Resources Carol McCoy 303-969-2096 [email protected] Division Ken Pierce Surficial Geologist, USGS–Bozeman 406-994-5085 [email protected] Supervisory GIS Specialist, Yellowstone Anne Rodman 307-344-7381 [email protected] National Park Shannon GIS Specialist, Yellowstone National Park 307-344-7381 [email protected] Savage Monday, May 16, involved a welcome to Yellowstone National Park and an introduction to the Geologic Resource Evaluation (GRE) Program, including status of reports and digital maps. -

6800-Year Vegetation and Fire History in the Bitterroot Mountain Range Montana
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1995 6800-year vegetation and fire history in the Bitterroot Mountain Range Montana Anne Elizabeth Karsian The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Karsian, Anne Elizabeth, "6800-year vegetation and fire history in the Bitterroot Mountain Range Montana" (1995). Graduate Student Theses, Dissertations, & Professional Papers. 6683. https://scholarworks.umt.edu/etd/6683 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. Anne- K a r s j a n i Maureen and Mike MANSFIELD LIBRARY TheM University ontana of Permission is granted by tlie author to reproduce this material in its entirety, provided that tliis material is used for scholarly purposes and is properly cited in published works and repoits. * * Please check "Yes'' or “No “ and provide signature Yes, I grant permission ..\1 No, I do not grant permission ----- Author’s Signature Date; ' n 1 Any copying for commercial purposes or financial gain may be undertaken only with ^he author’s explicit consent. ■ Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. A 6800-YEAR VEGETATION AND FIRE HISTORY IN THE BITTERROOT MOUNTAIN RANGE, MONTANA By ANNE ELIZABETH KARSIAN Presented in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE UNIVERSHY OF MONTANA Department of Forestry 1995 Approved by: t' z o Chairperson 7 ^ ^ ^ /. -

Landsat Evaluation of Trumpeter Swan Historical Nesting Sites In
Eastern Kentucky University Encompass Online Theses and Dissertations Student Scholarship 2014 Landsat Evaluation Of Trumpeter Swan Historical Nesting Sites In Yellowstone National Park Laura Elizabeth Cockrell Eastern Kentucky University, [email protected] Follow this and additional works at: https://encompass.eku.edu/etd Part of the Ecology and Evolutionary Biology Commons, and the Ornithology Commons Recommended Citation Cockrell, Laura Elizabeth, "Landsat Evaluation Of Trumpeter Swan Historical Nesting Sites In Yellowstone National Park" (2014). Online Theses and Dissertations. 222. https://encompass.eku.edu/etd/222 This Open Access Thesis is brought to you for free and open access by the Student Scholarship at Encompass. It has been accepted for inclusion in Online Theses and Dissertations by an authorized administrator of Encompass. For more information, please contact [email protected]. LANDSAT EVALUATION OF TRUMPETER SWAN HISTORICAL NESTING SITES IN YELLOWSTONE NATIONAL PARK By Laura Elizabeth Cockrell Bachelor of Science California State University, Chico Chico, California 2007 Submitted to the Faculty of the Graduate School of Eastern Kentucky University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May, 2014 Copyright © Laura Elizabeth Cockrell, 2014 All rights reserved ii DEDICATION This thesis is dedicated to my family and friends for their unwavering support during this adventure. iii ACKNOWLEDGMENTS This research was made possible through funding from the Yellowstone Park Foundation and the Society of Wetland Scientists Student Research Grant for support of field work, and by a Graduate Assistantship and Research Assistantship from the Department of Biological Sciences at Eastern Kentucky University. Thank you to Dr. Bob Frederick for his insight and persistence and for providing the GIS lab and to Dr. -
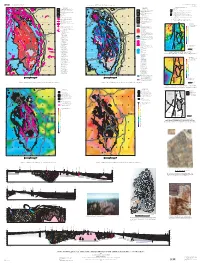
Map Showing Geology, Structure, and Geophysics of the Central Black
U.S. DEPARTMENT OF THE INTERIOR Prepared in cooperation with the SCIENTIFIC INVESTIGATIONS MAP 2777 U.S. GEOLOGICAL SURVEY SOUTH DAKOTA SCHOOL OF MINES AND TECHNOLOGY FOUNDATION SHEET 2 OF 2 Pamphlet accompanies map 104°00' 103°30' 103°00' 104°00' 103°30' 103°00' ° ° EXPLANATION FOR MAPS F TO H 44 30' 44°30' EXPLANATION 44 30' 44°30' EXPLANATION Spearfish Geologic features 53 54 Tertiary igneous rocks (Tertiary and post-Tertiary Spearfish PHANEROZOIC ROCKS 90 1 90 sedimentary rocks not shown) Pringle fault 59 Tertiary igneous rocks (Tertiary and post-Tertiary Pre-Tertiary and Cretaceous (post-Inyan Kara sedimentary rocks not shown) Monocline—BHM, Black Hills monocline; FPM, Fanny Peak monocline 52 85 Group) rocks 85 Sturgis Sturgis Pre-Tertiary and Cretaceous (post-Inyan Kara A Proposed western limit of Early Proterozoic rocks in subsurface 55 Lower Cretaceous (Inyan Kara Group), Jurassic, Group) rocks 57 58 60 14 and Triassic rocks 14 Lower Cretaceous (Inyan Kara Group), Jurassic, B Northern extension (fault?) of Fanny Peak monocline and Triassic rocks Paleozoic rocks C Possible eastern limit of Early Proterozoic rocks in subsurface 50 Paleozoic rocks Precambrian rocks S Possible suture in subsurface separating different tectonic terranes 89 51 89 2 PRECAMBRIAN ROCKS of Sims (1995) 49 Contact St 3 G Harney Peak Granite (unit Xh) Geographic features—BL, Bear Lodge Mountains; BM, Bear Mountain; Fault—Dashed where approximately located G DT DT, Devils Tower 48 B Early Proterozoic rocks, undivided Anticline—Showing trace of axial surface and 1 St Towns and cities—B, Belle Fourche; C, Custer; E, Edgemont; HS, Hot direction of plunge.