Catenin and APC Reveal Roles for Canonical Wnt Signaling in Lens Differentiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detailed Review Paper on Retinoid Pathway Signalling
1 1 Detailed Review Paper on Retinoid Pathway Signalling 2 December 2020 3 2 4 Foreword 5 1. Project 4.97 to develop a Detailed Review Paper (DRP) on the Retinoid System 6 was added to the Test Guidelines Programme work plan in 2015. The project was 7 originally proposed by Sweden and the European Commission later joined the project as 8 a co-lead. In 2019, the OECD Secretariat was added to coordinate input from expert 9 consultants. The initial objectives of the project were to: 10 draft a review of the biology of retinoid signalling pathway, 11 describe retinoid-mediated effects on various organ systems, 12 identify relevant retinoid in vitro and ex vivo assays that measure mechanistic 13 effects of chemicals for development, and 14 Identify in vivo endpoints that could be added to existing test guidelines to 15 identify chemical effects on retinoid pathway signalling. 16 2. This DRP is intended to expand the recommendations for the retinoid pathway 17 included in the OECD Detailed Review Paper on the State of the Science on Novel In 18 vitro and In vivo Screening and Testing Methods and Endpoints for Evaluating 19 Endocrine Disruptors (DRP No 178). The retinoid signalling pathway was one of seven 20 endocrine pathways considered to be susceptible to environmental endocrine disruption 21 and for which relevant endpoints could be measured in new or existing OECD Test 22 Guidelines for evaluating endocrine disruption. Due to the complexity of retinoid 23 signalling across multiple organ systems, this effort was foreseen as a multi-step process. -

Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-Like Mouse Models: Tracking the Role of the Hairless Gene
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2006 Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-like Mouse Models: Tracking the Role of the Hairless Gene Yutao Liu University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Life Sciences Commons Recommended Citation Liu, Yutao, "Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino- like Mouse Models: Tracking the Role of the Hairless Gene. " PhD diss., University of Tennessee, 2006. https://trace.tennessee.edu/utk_graddiss/1824 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Yutao Liu entitled "Molecular and Physiological Basis for Hair Loss in Near Naked Hairless and Oak Ridge Rhino-like Mouse Models: Tracking the Role of the Hairless Gene." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Life Sciences. Brynn H. Voy, Major Professor We have read this dissertation and recommend its acceptance: Naima Moustaid-Moussa, Yisong Wang, Rogert Hettich Accepted for the Council: Carolyn R. -

The Extracellular Matrix Phenome Across Species
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.06.980169; this version posted March 6, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. The extracellular matrix phenome across species Cyril Statzer1 and Collin Y. Ewald1* 1 Eidgenössische Technische Hochschule Zürich, Department of Health Sciences and Technology, Institute of Translational Medicine, Schwerzenbach-Zürich CH-8603, Switzerland *Corresponding authors: [email protected] (CYE) Keywords: Phenome, genotype-to-phenotype, matrisome, extracellular matrix, collagen, data mining. Highlights • 7.6% of the human phenome originates from variations in matrisome genes • 11’671 phenotypes are linked to matrisome genes of humans, mice, zebrafish, Drosophila, and C. elegans • Expected top ECM phenotypes are developmental, morphological and structural phenotypes • Nonobvious top ECM phenotypes include immune system, stress resilience, and age-related phenotypes 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.06.980169; this version posted March 6, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Abstract 2 Extracellular matrices are essential for cellular and organismal function. Recent 3 genome-wide and phenome-wide association studies started to reveal a broad 4 spectrum of phenotypes associated with genetic variants. However, the phenome or 5 spectrum of all phenotypes associated with genetic variants in extracellular matrix 6 genes is unknown. -
BSCB Newsletter 2017D
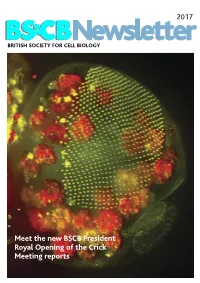
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

Original Article Parathyroid Hormone Induces Epithelial-To-Mesenchymal Transition Via the Wnt/Β-Catenin Signaling Pathway in Human Renal Proximal Tubular Cells
Int J Clin Exp Pathol 2014;7(9):5978-5987 www.ijcep.com /ISSN:1936-2625/IJCEP0001621 Original Article Parathyroid hormone induces epithelial-to-mesenchymal transition via the Wnt/β-catenin signaling pathway in human renal proximal tubular cells Yunshan Guo1*, Zhen Li1*, Raohai Ding1, Hongdong Li1, Lei Zhang1, Weijie Yuan2, Yanxia Wang1 1Department of Nephrology, General Hospital of Ji’nan Military Command, Ji’nan 250031, China; 2Department of Nephrology, Shanghai Jiaotong University Affiliated First People’s Hospital, 85 Wu Jin Road, Shanghai 200080, China. *Equal contributors. Received July 30, 2014; Accepted August 21, 2014; Epub August 15, 2014; Published September 1, 2014 Abstract: Epithelial-to-mesenchymal transition (EMT) has been shown to play an important role in renal fibrogen- esis. Recent studies suggested parathyroid hormone (PTH) could accelerate EMT and subsequent organ fibrosis. However, the precise molecular mechanisms underlying PTH-induced EMT remain unknown. The present study was to investigate whether Wnt/β-catenin signaling pathway is involved in PTH-induced EMT in human renal proximal tubular cells (HK-2 cells) and to determine the profile of gene expression associated with PTH-induced EMT. PTH could induce morphological changes and gene expression characteristic of EMT in cultured HK-2 cells. Suppressing β-catenin expression or DKK1 limited gene expression characteristic of PTH-induced EMT. Based on the PCR array analysis, PTH treatment resulted in the up-regulation of 18 genes and down-regulation of 9 genes compared with the control. The results were further supported by a western blot analysis, which showed the increased Wnt4 protein expression. Wnt4 overexpression also promotes PTH-induced EMT in HK-2 cells. -

A Commentary on WNT7A Implication in Cervical Cancer Development
ndrom Sy es tic & e G n e e n G e f T o Artaza-Irigaray et al., J Genet Syndr Gene Ther 2015, 6:3 Journal of Genetic Syndromes h l e a r n a DOI: 10.4172/2157-7412.1000267 r p u y o J & Gene Therapy ISSN: 2157-7412 Commentary Open Access A Commentary on WNT7A Implication in Cervical Cancer Development Cristina Artaza-Irigaray1,2, Adriana Aguilar-Lemarroy1 and Luis F Jave-Suárez1* 1División de Inmunología, Centro de Investigación Biomédica de Occidente (CIBO), Instituto Mexicano del Seguro Social (IMSS), Guadalajara, Jalisco, Mexico 2Programa de Doctorado en Ciencias Biomédicas, Centro Universitario de Ciencias de la Salud (CUCS) - Universidad de Guadalajara, Jalisco, Mexico Cervical Cancer (CC) is the fourth leading cause of cancer Conversely, silencing Wnt7a in HaCaT cells induced an increase in cell deaths in women worldwide and is associated directly with Human proliferation and migration rates. These results suggest that the loss of papillomavirus (HPV) infection [1]. Many authors have reported Wnt7a expression probably contributes to increased cell proliferation that HPV can immortalize human cells without leading to cell and migration during cervical tumor development. transformation by itself [2,3]. Thus, cervical carcinogenesis is a As responses always lead to new questions, the next step was multistep process involving HPV infection and additional alterations. to elucidate the way in which Wnt7a ligand expression was being In 2005, canonical Wnt signaling pathway activation was proposed repressed. Wnt7a is known to possess tumor suppressor properties as a second hit during epithelial malignant transformation but this in several cancers and is frequently inactivated due to CpG-island hypothesis remains controversial [3,4]. -

WNT11-Conditioned Medium Promotes Angiogenesis Through the Activation of Non-Canonical WNT-PKC-JNK Signaling Pathway
G C A T T A C G G C A T genes Article WNT11-Conditioned Medium Promotes Angiogenesis through the Activation of Non-Canonical WNT-PKC-JNK Signaling Pathway § Jingcai Wang y, Min Gong z, Shi Zuo , Jie Xu, Chris Paul, Hongxia Li k, Min Liu, Yi-Gang Wang, Muhammad Ashraf ¶ and Meifeng Xu * Department of Pathology and Laboratory Medicine, University of Cincinnati Medical Center, Cincinnati, OH 45267, USA; [email protected] (J.W.); [email protected] (M.G.); [email protected] (S.Z.); [email protected] (J.X.); [email protected] (C.P.); [email protected] (H.L.); [email protected] (M.L.); [email protected] (Y.-G.W.); [email protected] (M.A.) * Correspondence: [email protected] Current address: Department of Pathology and Laboratory Medicine, Nationwide Children’s Hospital, y Columbus, OH 43205, USA. Current Address: Department of Neonatology, Children’s Hospital of Soochow University, z Suzhou 215025, Jiangsu, China. § Current Address: Department of Hepatobiliary Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang 550025, Guizhou, China. Current Address: Department of Cardiology, The First Affiliated Hospital of Soochow University, k Suzhou 215006, Jiangsu, China. ¶ Current Address: Department of Medicine, Cardiology, Medical College of Georgia, Augusta University, Augusta, GA 30912, USA. Received: 10 August 2020; Accepted: 26 October 2020; Published: 29 October 2020 Abstract: Background: We demonstrated that the transduction of Wnt11 into mesenchymal stem cells (MSCs) (MSCWnt11) promotes these cells differentiation into cardiac phenotypes. In the present study, we investigated the paracrine effects of MSCWnt11 on cardiac function and angiogenesis. -

A High-Fat/High-Sucrose Diet Induces WNT4 Expression in Mouse Pancreatic Β-Cells
This is “Advance Publication Article” Kurume Medical Journal, 65, 00-00, 2018 Original Article A High-Fat/High-Sucrose Diet Induces WNT4 Expression in Mouse Pancreatic β-cells YAYOI KURITA, TSUYOSHI OHKI, ERI SOEJIMA, XIAOHONG YUAN, SATOMI KAKINO, NOBUHIKO WADA, TOSHIHIKO HASHINAGA, HITOMI NAKAYAMA, JUNICHI TANI, YUJI TAJIRI, YUJI HIROMATSU, KENTARO YAMADA* AND MASATOSHI NOMURA Division of Endocrinology and Metabolism, Department of Internal Medicine, Kurume University School of Medicine, Kurume 830-0011, Japan, *Diabetes Center of Asakura Medical Association Hospital, Asakura 838-0069, Japan Received 12 February 2018, accepted 1 May 2018 J-STAGE advance publication 11 March 2019 Edited by MAKOTO TAKANO Summary: Aims/Introduction: Several lines of evidence suggest that dysregulation of the WNT signaling pathway is involved in the pathogenesis of type 2 diabetes. This study was performed to elucidate the effects of a high-fat/high-sucrose (HF/HS) diet on pancreatic islet functions in relation to modulation of WNT ligand expression in β-cells. Materials and Methods: Mice were fed either standard mouse chow or a HF/HS diet from 8 weeks of age. At 20 weeks of age, intraperitoneal glucose tolerance tests were performed in both groups of mice, followed by euthanasia and isolation of pancreatic islets. WNT-related gene expression in islets and MIN6 cells was measured by quantitative real-time RT-PCR. To explore the direct effects of WNT signals on pancreatic β-cells, MIN6 cells were exposed to recombinant mouse WNT4 protein (rmWNT4) for 48 h, and glucose-induced insulin secre- tion was measured. Furthermore, Wnt4 siRNAs were transfected into MIN6 cells, and cell viability and insulin secretion were measured in control and Wnt4 siRNA-transfected MIN6 cells. -

WNT10A Gene Wnt Family Member 10A
WNT10A gene Wnt family member 10A Normal Function The WNT10A gene is part of a large family of WNT genes, which play critical roles in development starting before birth. These genes provide instructions for making proteins that participate in chemical signaling pathways in the body. Wnt signaling controls the activity of certain genes and regulates the interactions between cells during embryonic development. The protein produced from the WNT10A gene plays a role in the development of many parts of the body. It appears to be essential for the formation of tissues that arise from an embryonic cell layer called the ectoderm. These tissues include the skin, hair, nails, teeth, and sweat glands. Researchers believe that the WNT10A protein is particularly important for the formation and shaping of both baby (primary) teeth and adult ( permanent) teeth. Health Conditions Related to Genetic Changes Hypohidrotic ectodermal dysplasia Several mutations in the WNT10A gene have been found to cause hypohidrotic ectodermal dysplasia, the most common form of ectodermal dysplasia. Starting before birth, ectodermal dysplasias result in the abnormal development of the skin, hair, nails, teeth, and sweat glands. Hypohidrotic ectodermal dysplasia is characterized by a reduced ability to sweat (hypohidrosis), sparse scalp and body hair (hypotrichosis), and several missing teeth (hypodontia) or teeth that are malformed. WNT10A gene mutations account for about 5 percent of all cases of hypohidrotic ectodermal dysplasia. Most of the WNT10A gene mutations associated with hypohidrotic ectodermal dysplasia change single protein building blocks (amino acids) in the WNT10A protein, which impairs its function. The resulting shortage of functional WNT10A protein disrupts Wnt signaling during the development of ectodermal tissues, particularly the teeth. -

Theranostics WNT6 Is a Novel Oncogenic Prognostic Biomarker In
Theranostics 2018, Vol. 8, Issue 17 4805 Ivyspring International Publisher Theranostics 2018; 8(17): 4805-4823. doi: 10.7150/thno.25025 Research Paper WNT6 is a novel oncogenic prognostic biomarker in human glioblastoma Céline S. Gonçalves1,2, Joana Vieira de Castro1,2, Marta Pojo1,2, Eduarda P. Martins1,2, Sandro Queirós1,2, Emmanuel Chautard3,4, Ricardo Taipa5, Manuel Melo Pires5, Afonso A. Pinto6, Fernando Pardal7, Carlos Custódia8, Cláudia C. Faria8,9, Carlos Clara10, Rui M. Reis1,2,10, Nuno Sousa1,2, Bruno M. Costa1,2 1. Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Campus Gualtar, 4710-057 Braga, Portugal 2. ICVS/3B’s - PT Government Associate Laboratory, Braga/Guimarães, Portugal 3. Université Clermont Auvergne, INSERM, U1240 IMoST, 63000 Clermont Ferrand, France 4. Pathology Department, Université Clermont Auvergne, Centre Jean Perrin, 63011 Clermont-Ferrand, France 5. Neuropathology Unit, Department of Neurosciences, Centro Hospitalar do Porto, Porto, Portugal 6. Department of Neurosurgery, Hospital Escala Braga, Sete Fontes - São Victor 4710-243 Braga, Portugal 7. Department of Pathology, Hospital Escala Braga, Sete Fontes - São Victor 4710-243 Braga, Portugal 8. Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal 9. Neurosurgery Department, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte (CHLN), Lisbon, Portugal 10. Molecular Oncology Research Center, Barretos Cancer Hospital, Barretos - S. Paulo, Brazil. Corresponding author: Bruno M. Costa, Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. Email: [email protected]; Phone: (+351)253604837; Fax: (+351)253604831 © Ivyspring International Publisher. -

Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response
International Journal of Molecular Sciences Review Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response Casey D. Stefanski 1,2 and Jenifer R. Prosperi 1,2,3,* 1 Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46617, USA; [email protected] 2 Mike and Josie Harper Cancer Research Institute, South Bend, IN 46617, USA 3 Department of Biochemistry and Molecular Biology, Indiana University School of Medicine-South Bend, South Bend, IN 46617, USA * Correspondence: [email protected]; Tel.: +1-574-631-4002 Received: 30 September 2020; Accepted: 20 October 2020; Published: 22 October 2020 Abstract: Resistance to chemotherapy occurs through mechanisms within the epithelial tumor cells or through interactions with components of the tumor microenvironment (TME). Chemoresistance and the development of recurrent tumors are two of the leading factors of cancer-related deaths. The Adenomatous Polyposis Coli (APC) tumor suppressor is lost in many different cancers, including colorectal, breast, and prostate cancer, and its loss correlates with a decreased overall survival in cancer patients. While APC is commonly known for its role as a negative regulator of the WNT pathway, APC has numerous binding partners and functional roles. Through APC’s interactions with DNA repair proteins, DNA replication proteins, tubulin, and other components, recent evidence has shown that APC regulates the chemotherapy response in cancer cells. In this review article, we provide an overview of some of the cellular processes in which APC participates and how they impact chemoresistance through both epithelial- and TME-derived mechanisms. Keywords: adenomatous polyposis coli; chemoresistance; WNT signaling 1. -

Feedback Interactions Between Cell–Cell Adherens Junctions and Cytoskeletal Dynamics in Newt Lung Epithelial Cells□V Clare M
Molecular Biology of the Cell Vol. 11, 2471–2483, July 2000 Feedback Interactions between Cell–Cell Adherens Junctions and Cytoskeletal Dynamics in Newt Lung Epithelial Cells□V Clare M. Waterman-Storer,*†‡ Wendy C. Salmon,†‡ and E.D. Salmon‡ *Department of Cell Biology and Institute for Childhood and Neglected Diseases, The Scripps Research Institute, La Jolla, California 92037; and ‡Department of Biology, University of North Carolina, Chapel Hill, North Carolina 27599 Submitted February 3, 2000; Revised April 20, 2000; Accepted May 11, 2000 Monitoring Editor: Jennifer Lippincott-Schwartz To test how cell–cell contacts regulate microtubule (MT) and actin cytoskeletal dynamics, we examined dynamics in cells that were contacted on all sides with neighboring cells in an epithelial cell sheet that was undergoing migration as a wound-healing response. Dynamics were recorded using time-lapse digital fluorescence microscopy of microinjected, labeled tubulin and actin. In fully contacted cells, most MT plus ends were quiescent; exhibiting only brief excursions of growth and shortening and spending 87.4% of their time in pause. This contrasts MTs in the lamella of migrating cells at the noncontacted leading edge of the sheet in which MTs exhibit dynamic instability. In the contacted rear and side edges of these migrating cells, a majority of MTs were also quiescent, indicating that cell–cell contacts may locally regulate MT dynamics. Using photoactivation of fluorescence techniques to mark MTs, we found that MTs in fully contacted cells did not undergo retrograde flow toward the cell center, such as occurs at the leading edge of motile cells. Time-lapse fluorescent speckle microscopy of fluorescently labeled actin in fully contacted cells revealed that actin did not flow rearward as occurs in the leading edge lamella of migrating cells.