Master Precertification List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Single Cell Mutational Profiling and Clonal Phylogeny in Cancer Nicola E
Downloaded from genome.cshlp.org on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press For GENOME RESEARCH (Methods) Single cell mutational profiling and clonal phylogeny in cancer Nicola E Potter – Institute of Cancer Research, UK Luca Ermini – Institute of Cancer Research, UK Elli Papaemmanuil – Wellcome Trust Sanger Institute, UK Giovanni Cazzaniga - Clinica Pediatrica, Italy Gowri Vijayaraghavan – Institute of Cancer Research, UK Ian Titley – Institute of Cancer Research, UK Anthony Ford – Institute of Cancer Research, UK Peter Campbell - Wellcome Trust Sanger Institute, UK Lyndal Kearney – Institute of Cancer Research, UK Mel Greaves – Institute of Cancer Research, UK Corresponding Author - Professor Mel Greaves FRS, Head, Haemato-Oncology Research Unit, Division of Molecular Pathology, Brookes Lawley Building, 15 Cotswold Road, Sutton, Surrey SM2 5NG T +44 (0)20 8722 4073 (direct) E [email protected] Running title: Genetic analysis of single cancer cells Keywords: Heterogeneity, Single cells, Cancer, Phylogeny 1 Downloaded from genome.cshlp.org on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press ABSTRACT The development of cancer is a dynamic evolutionary process in which intra-clonal, genetic diversity provides a substrate for clonal selection and a source of therapeutic escape. The complexity and topography of intra-clonal genetic architecture has major implications for biopsy-based prognosis and for targeted therapy. High depth, next generation sequencing (NGS) efficiently captures the mutational load of individual tumours or biopsies. But, being a snapshot portrait of total DNA, it disguises the fundamental features of sub-clonal variegation of genetic lesions and of clonal phylogeny. Single cell genetic profiling provides a potential resolution to this problem but methods developed to date all have limitations. -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

COMMENTARY Does Transcription by RNA Polymerase Play a Direct Role in the Initiation of Replication?
Journal of Cell Science 107, 1381-1387 (1994) 1381 Printed in Great Britain © The Company of Biologists Limited 1994 COMMENTARY Does transcription by RNA polymerase play a direct role in the initiation of replication? A. Bassim Hassan* and Peter R. Cook† CRC Nuclear Structure and Function Research Group, Sir William Dunn School of Pathology, University of Oxford, South Parks Road, Oxford, UK *Present address: Addenbrooke’s NHS Trust, Hills Rd, Cambridge CB2 2QQ, UK †Author for correspondence SUMMARY RNA polymerases have been implicated in the initiation of replication in bacteria. The conflicting evidence for a role in initiation in eukaryotes is reviewed. Key words: cell cycle, initiation, origin, replication, transcription PRIMERS AND PRIMASES complex becomes exclusively involved in the synthesis of Okazaki fragments on the lagging strand. A critical step in this DNA polymerases cannot initiate the synthesis of new DNA process is the unwinding of the duplex. chains, they can only elongate pre-existing primers. The opposite polarities of the two strands of the double helix coupled with the 5′r3′ polarity of the polymerase means that RNA POLYMERASES AND THE INITIATION OF replication occurs relatively continuously on one (leading) REPLICATION IN BACTERIA strand and discontinuously on the other (lagging) strand. The continuous strand probably needs to be primed once, usually The first evidence of a role for RNA polymerase came from at an origin. Nature has found many different ways of doing the demonstration that the initiation of replication in this, including the use of RNA primers made by an RNA poly- Escherichia coli was sensitive to rifampicin, an inhibitor of merase (e.g. -

Antibiotic Resistance by High-Level Intrinsic Suppression of a Frameshift Mutation in an Essential Gene
Antibiotic resistance by high-level intrinsic suppression of a frameshift mutation in an essential gene Douglas L. Husebya, Gerrit Brandisa, Lisa Praski Alzrigata, and Diarmaid Hughesa,1 aDepartment of Medical Biochemistry and Microbiology, Biomedical Center, Uppsala University, Uppsala SE-751 23, Sweden Edited by Sankar Adhya, National Institutes of Health, National Cancer Institute, Bethesda, MD, and approved January 4, 2020 (received for review November 5, 2019) A fundamental feature of life is that ribosomes read the genetic (unlikely if the DNA sequence analysis was done properly), that the code in messenger RNA (mRNA) as triplets of nucleotides in a mutants carry frameshift suppressor mutations (15–17), or that the single reading frame. Mutations that shift the reading frame gen- mRNA contains sequence elements that promote a high level of ri- erally cause gene inactivation and in essential genes cause loss of bosomal shifting into the correct reading frame to support cell viability viability. Here we report and characterize a +1-nt frameshift mutation, (10). Interest in understanding these mutations goes beyond Mtb and centrally located in rpoB, an essential gene encoding the beta-subunit concerns more generally the potential for rescue of mutants that ac- of RNA polymerase. Mutant Escherichia coli carrying this mutation are quire a frameshift mutation in any essential gene. We addressed this viable and highly resistant to rifampicin. Genetic and proteomic exper- by isolating a mutant of Escherichia coli carrying a frameshift mutation iments reveal a very high rate (5%) of spontaneous frameshift suppres- in rpoB and experimentally dissecting its genotype and phenotypes. sion occurring on a heptanucleotide sequence downstream of the mutation. -

Expression of the POTE Gene Family in Human Ovarian Cancer Carter J Barger1,2, Wa Zhang1,2, Ashok Sharma 1,2, Linda Chee1,2, Smitha R
www.nature.com/scientificreports OPEN Expression of the POTE gene family in human ovarian cancer Carter J Barger1,2, Wa Zhang1,2, Ashok Sharma 1,2, Linda Chee1,2, Smitha R. James3, Christina N. Kufel3, Austin Miller 4, Jane Meza5, Ronny Drapkin 6, Kunle Odunsi7,8,9, 2,10 1,2,3 Received: 5 July 2018 David Klinkebiel & Adam R. Karpf Accepted: 7 November 2018 The POTE family includes 14 genes in three phylogenetic groups. We determined POTE mRNA Published: xx xx xxxx expression in normal tissues, epithelial ovarian and high-grade serous ovarian cancer (EOC, HGSC), and pan-cancer, and determined the relationship of POTE expression to ovarian cancer clinicopathology. Groups 1 & 2 POTEs showed testis-specifc expression in normal tissues, consistent with assignment as cancer-testis antigens (CTAs), while Group 3 POTEs were expressed in several normal tissues, indicating they are not CTAs. Pan-POTE and individual POTEs showed signifcantly elevated expression in EOC and HGSC compared to normal controls. Pan-POTE correlated with increased stage, grade, and the HGSC subtype. Select individual POTEs showed increased expression in recurrent HGSC, and POTEE specifcally associated with reduced HGSC OS. Consistent with tumors, EOC cell lines had signifcantly elevated Pan-POTE compared to OSE and FTE cells. Notably, Group 1 & 2 POTEs (POTEs A/B/B2/C/D), Group 3 POTE-actin genes (POTEs E/F/I/J/KP), and other Group 3 POTEs (POTEs G/H/M) show within-group correlated expression, and pan-cancer analyses of tumors and cell lines confrmed this relationship. Based on their restricted expression in normal tissues and increased expression and association with poor prognosis in ovarian cancer, POTEs are potential oncogenes and therapeutic targets in this malignancy. -
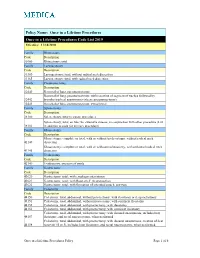
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Oncogene Associated Cdna Microarray Analysis Shows PRAME
J Clin Exp Hematopathol Vol. 49, No. 1, May 2009 Original Article Oncogene Associated cDNA Microarray Analysis Shows PRAME Gene Expression is a Marker for Response to Anthracycline Containing Chemotherapy in Patients with Diffuse Large B-cell Lymphoma Riko Kawano,1,3) Kennosuke Karube,3) Masahiro Kikuchi,2) Morishige Takeshita,2) Kazuo Tamura,1) Naokuni Uike,4) Tetsuya Eto,5) Koichi Ohshima,3) and Junji Suzumiya1,6) CHOP (cyclophosphamide, adriamycin, vincristine, and prednisolone) therapy achieves a response in more than 60% patients with diffuse large B-cell lymphomas (DLBCLs). However, DLBCL shows a heterogeneous response to chemotherapy, and some patients are refractory to CHOP therapy. This difference in response to therapy is most likely due to differences in biological characteristics. We used cDNA microarray analysis to identify genes differentially expressed in anthracycline containing chemotherapy-resistant DLBCLs (7 patients) compared with anthracycline containing chemotherapy-sensitive DLBCLs (6 patients). Nine genes on the cDNA chip showed increased expression in anthracycline containing chemotherapy- resistant patients. We chose the preferentially expressed antigen of melanoma (PRAME) gene because it showed the highest expression in anthracycline containing chemotherapy-resistant DLBCLs on the cDNA chip, and it has been linked to prognosis of hematological malignancies. We also examined the relationship between PRAME gene expression and progression-free survival (PFS) in 45 patients with DLBCL. The progression-free survival -

Resistance to Rifampicin: a Review
The Journal of Antibiotics (2014) 67, 625–630 & 2014 Japan Antibiotics Research Association All rights reserved 0021-8820/14 www.nature.com/ja REVIEW ARTICLE Resistance to rifampicin: a review Beth P Goldstein Resistance to rifampicin (RIF) is a broad subject covering not just the mechanism of clinical resistance, nearly always due to a genetic change in the b subunit of bacterial RNA polymerase (RNAP), but also how studies of resistant polymerases have helped us understand the structure of the enzyme, the intricacies of the transcription process and its role in complex physiological pathways. This review can only scratch the surface of these phenomena. The identification, in strains of Escherichia coli, of the positions within b of the mutations determining resistance is discussed in some detail, as are mutations in organisms that are therapeutic targets of RIF, in particular Mycobacterium tuberculosis. Interestingly, changes in the same three codons of the consensus sequence occur repeatedly in unrelated RIF-resistant (RIFr) clinical isolates of several different bacterial species, and a single mutation predominates in mycobacteria. The utilization of our knowledge of these mutations to develop rapid screening tests for detecting resistance is briefly discussed. Cross-resistance among rifamycins has been a topic of controversy; current thinking is that there is no difference in the susceptibility of RNAP mutants to RIF, rifapentine and rifabutin. Also summarized are intrinsic RIF resistance and other resistance mechanisms. The Journal of Antibiotics (2014) 67, 625–630; doi:10.1038/ja.2014.107; published online 13 August 2014 INTRODUCTION laboratory studies and emerging in patients who received mono- In celebrating the life of Professor Piero Sensi and his discovery of therapy with RIF. -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

Short-Term Western-Style Diet Negatively Impacts Reproductive Outcomes in Primates
Short-term Western-style diet negatively impacts reproductive outcomes in primates Sweta Ravisankar, … , Shawn L. Chavez, Jon D. Hennebold JCI Insight. 2021;6(4):e138312. https://doi.org/10.1172/jci.insight.138312. Research Article Metabolism Reproductive biology A maternal Western-style diet (WSD) is associated with poor reproductive outcomes, but whether this is from the diet itself or underlying metabolic dysfunction is unknown. Here, we performed a longitudinal study using regularly cycling female rhesus macaques (n = 10) that underwent 2 consecutive in vitro fertilization (IVF) cycles, one while consuming a low-fat diet and another 6–8 months after consuming a high-fat WSD. Metabolic data were collected from the females prior to each IVF cycle. Follicular fluid (FF) and oocytes were assessed for cytokine/steroid levels and IVF potential, respectively. Although transition to a WSD led to weight gain and increased body fat, no difference in insulin levels was observed. A significant decrease in IL-1RA concentration and the ratio of cortisol/cortisone was detected in FF after WSD intake. Despite an increased probability of isolating mature oocytes, a 44% reduction in blastocyst number was observed with WSD consumption, and time-lapse imaging revealed delayed mitotic timing and multipolar divisions. RNA sequencing of blastocysts demonstrated dysregulation of genes involved in RNA binding, protein channel activity, mitochondrial function and pluripotency versus cell differentiation after WSD consumption. Thus, short-term WSD consumption promotes a proinflammatory intrafollicular microenvironment that is associated with impaired preimplantation development in the absence of large-scale metabolic changes. Find the latest version: https://jci.me/138312/pdf RESEARCH ARTICLE Short-term Western-style diet negatively impacts reproductive outcomes in primates Sweta Ravisankar,1,2 Alison Y.