Durvalumab Monotherapy As a Third-Line Treatment for Extensive-Stage Small-Cell Lung Cancer: a Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatial Distribution Pattern of Minshuku in the Urban Agglomeration of Yangtze River Delta
The Frontiers of Society, Science and Technology ISSN 2616-7433 Vol. 3, Issue 1: 23-35, DOI: 10.25236/FSST.2021.030106 Spatial Distribution Pattern of Minshuku in the Urban Agglomeration of Yangtze River Delta Yuxin Chen, Yuegang Chen Shanghai University, Shanghai 200444, China Abstract: The city cluster in Yangtze River Delta is the core area of China's modernization and economic development. The industry of Bed and Breakfast (B&B) in this area is relatively developed, and the distribution and spatial pattern of Minshuku will also get much attention. Earlier literature tried more to explore the influence of individual characteristics of Minshuku (such as the design style of Minshuku, etc.) on Minshuku. However, the development of Minshuku has a cluster effect, and the distribution of domestic B&Bs is very unbalanced. Analyzing the differences in the distribution of Minshuku and their causes can help the development of the backward areas and maintain the advantages of the developed areas in the industry of Minshuku. This article finds that the distribution of Minshuku is clustered in certain areas by presenting the overall spatial distribution of Minshuku and cultural attractions in Yangtze River Delta and the respective distribution of 27 cities. For example, Minshuku in the central and eastern parts of Yangtze River Delta are more concentrated, so are the scenic spots in these areas. There are also several concentrated Minshuku areas in other parts of Yangtze River Delta, but the number is significantly less than that of the central and eastern regions. Keywords: Minshuku, Yangtze River Delta, Spatial distribution, Concentrated distribution 1. -

Annual Report 2007 3 Live Demonstration of Beijing Majestic Mansion Ultimate Grace of Living Corporate Profile
The homes built by Greentown lead lifestyle. Our premier class of architecture fully demonstrates dynamic blend of taste and culture. The architecture characteristics embrace the culture of city and show respect to natural landscape. Join us to live elegantly and delicately. Since its establishment, Greentown is determined to create beauty for the city with an idealistic human-oriented spirit adopted through the course of development and after-sales services for its property products, and bring ideal life for its customers with quality properties. Contents Corporate Information 3 Corporate Profile 6 Portfolio 8 Year in Review 44 Chairman’s Statement 48 CEO’s Review 49 Management Discussion and Analysis 58 Directors and Senior Management 74 Corporate Governance Report 84 Report of the Directors 90 Report of the Auditors 99 Consolidated Income Statement 101 Consolidated Balance Sheet 102 Consolidated Statement of Changes in Equity 104 Consolidated Cash Flow Statement 105 Notes to the Consolidated Financial Statements 107 Five Year Financial Summary 201 Valuation Report and Analysis 202 Corporate Information Directors Remuneration Committee Legal Advisors to Our Company Executive Directors Mr. JIA Shenghua as to Hong Kong law and U.S. law: Mr. SONG Weiping (Chairman) Mr. SZE Tsai Ping, Michael Herbert Smith Mr. SHOU Bainian Mr. CHEN Shunhua (Executive Vice-Chairman) as to PRC law: Mr. CHEN Shunhua Nomination Committee Zhejiang T&C Law Firm Mr. GUO Jiafeng Mr. SZE Tsai Ping, Michael Mr. TSUI Yiu Wa, Alec as to Cayman Islands law and Independent Non-Executive Directors Mr. SHOU Bainian British Virgin Islands law: Maples and Calder Mr. JIA Shenghua Mr. -

Multiple Lineages of Dengue Virus Serotype 2 Cosmopolitan Genotype
www.nature.com/scientificreports OPEN Multiple Lineages of Dengue Virus Serotype 2 Cosmopolitan Genotype Caused a Local Dengue Outbreak Received: 13 August 2018 Accepted: 26 April 2019 in Hangzhou, Zhejiang Province, Published: xx xx xxxx China, in 2017 Hua Yu1, Qingxin Kong2, Jing Wang2, Xiaofeng Qiu1, Yuanyuan Wen2, Xinfen Yu1, Muwen Liu2, Haoqiu Wang1, Jingcao Pan1 & Zhou Sun2 During July to November 2017, a large dengue outbreak involving 1,138 indigenous cases occurred in Hangzhou, Zhejiang Province, China. All patients were clinically diagnosed as mild dengue. Epidemiology investigation and phylogenetic analysis of circulating viruses revealed that at least three lineages of dengue virus serotype 2 (DENV-2) Cosmopolitan genotype initiated the outbreak during a short time. The analysis of the time to most recent common ancestor estimated that the putative ancestor of these DENV-2 lineages might rise no later than March, 2017, suggesting independent introductions of these lineages into Hangzhou. We presumed that group travelers visiting dengue- endemic areas gave rise to multiple introductions of these lineages during so short a time. Co-circulating of multiple DENV-2 lineages, emerging of disease in urban areas, hot and humid weather in Hangzhou adequate for mosquito breeding, and limited dengue diagnosis abilities of local hospitals, were the reasons causing the large local outbreak in Hangzhou. Dengue is a mosquito-borne infectious disease caused by dengue viruses (DENVs) which consists of four anti- genically distinct but closely related serotypes (DENV-1, DENV-2, DENV-3, and DENV-4). Dengue prevails in tropical and sub-tropical regions worldwide. It has been estimated recently that a total of 58.40 million sympto- matic dengue viral infections, including 13.57 thousand fatal cases, occurred globally in 2013 (ref.1). -

Tian Ge Interactive Holdings Limited 天鴿互動控股有限公司 (Incorporated in the Cayman Islands with Limited Liability) (Stock Code: 1980)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. Tian Ge Interactive Holdings Limited 天鴿互動控股有限公司 (Incorporated in the Cayman Islands with limited liability) (Stock code: 1980) ANNOUNCEMENT OF ANNUAL RESULTS FOR THE YEAR ENDED DECEMBER 31, 2019 FINANCIAL HIGHLIGHTS Year ended December 31, Change (in RMB’000) 2019 2018 % Revenue 539,329 751,933 -28.3% – Online interactive entertainment service 442,814 634,159 -30.2% – Advertising services 72,144 64,286 12.2% – Others 24,371 53,488 -54.4% Gross profit 474,784 686,643 -30.9% Gross profit margin 88.0% 91.3% Net profit 100,126 215,662 -53.6% Net profit margin 18.6% 28.7% Earnings per share (expressed in RMB per share) – basic 0.075 0.172 -56.4% – diluted 0.074 0.168 -56.0% Adjusted net profit(1) 181,236 342,471 -47.1% Adjusted net profit margin(2) 33.6% 45.5% Adjusted EBITDA(3) 298,949 434,842 -31.3% Adjusted EBITDA margin 55.4% 57.8% Total assets 3,502,764 3,156,540 11.0% Total liabilities 638,021 312,370 104.3% 1 Notes: (1) Adjusted net profit was derived from the net profit for the period excluding the effect of non-cash share-based compensation expenses, net losses/(gains) from investee companies, impairment provision, amortization of intangible assets arising from acquisitions and income tax effects of non-IFRS adjustments. -

Monitoring Ground Subsidence in Areas Covered by Dense Vegetation Using Terrasar-X Images: a Case Study of Hangzhou
The International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume XLI-B7, 2016 XXIII ISPRS Congress, 12–19 July 2016, Prague, Czech Republic MONITORING GROUND SUBSIDENCE IN AREAS COVERED BY DENSE VEGETATION USING TERRASAR-X IMAGES: A CASE STUDY OF HANGZHOU H. A. Wu a, *, Y. H. Zhanga , G. F. Luo b, Y. K. Kanga , Y. M. Zhu b a Chinese Academy of Surveying and Mapping, 100830 Haidian District, Beijing, China - (wuha, yhzhang) @casm.ac.cn, [email protected], b Zhejiang Academy of Surveying and Mapping, 310012 Xihu District, Hangzhou, China - [email protected], [email protected] Commission VII, WG VII/2 KEY WORDS: Ground subsidence, InSAR, high resolution, TerraSAR-X, Hangzhou ABSTRACT: Hangzhou, the capital of Zhejiang province has suffered serious ground subsidence during the past several decades, due to long term over-exploration of groundwater. In this paper, the time series InSAR technique using high resolution SAR images is investigated for the generation of subsidence maps over Hangzhou region. 29 TerraSAR-X images acquired from May 2012 to Sep 2015 are used. The results show that serious subsidence has mainly taken place in suburban area, including Yuhang district, Xiaoshan district and Binjiang district. 4 subsidence centers are discovered, namely Tangqi town in Yuhang with an average subsiding velocity of -29.6 mm/year, Xintang (-30.7 mm/year) in Xiaoshan, Zhujiaqiao town (-25.6mm/year) in Xiaoshan, and Miaohouwang town (- 30.1mm/year) in Binjiang. The urban area is stable and ground rebound even take place in some places. The results are compared with 19 levelling measurements. -
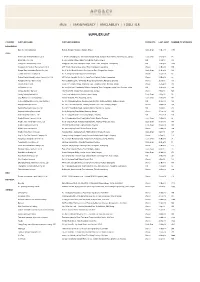
Traceability | Together
TRUST | TRANSPARENCY | TRACEABILITY | TOGETHER SUPPLIER LIST COUNTRY SUPPLIER NAME SUPPLIER ADDRESS PRODUCTS LAST AUDIT NUMBER OF WORKERS BANGLEDESH Basicline International Ltd Bokran, Monipur, Mirzapur, Gazipur, Dhaka Cut & Sewn 13-Dec-18 1198 CHINA Binhai Clear Knitted Fashion Co., Ltd. 2~3F Shixun Building, No. 199, North Zhongshi Road, Dongkan Town, Binhai, Yancheng City, Jiangsu Cut & Sewn 12-Sep-18 86 Binhai Sales Co., Ltd Section 2, Batan Village, Batan Town, Binhai Coutry, Jiangsu Knit 31-Jul-18 338 Dongguan Cason Knitting Co Ltd Xiangyuan East Road, Shangtun Village, Liaobu Town, Dongguan , Guangdong Knit 14-Aug-18 1068 Dongguan Xin Neng Jin He Garment Co Ltd #1B, Yinghu Industrial Area, Qingxi Town, Dongguan, Guangdong Woven 27-Nov-18 100 Dongtai Sumeng Knitting Fashion Co., Ltd. No.11 Weft 2 Road, Economic Development Zone, Dongtai City, Jiangsu Cut & Sewn 18-Sep-18 223 Eurohai Garment Company Ltd. No.36 Tian peng Road,Fengxian Area,Shanghai Woven 15-Oct-18 37 Foshan Nanhai Shengli Leather Garment Co., Ltd. 4/F No.358, Jiang Bin Xin Qu, Jiu Jiang Town, Nanhai, Foshan, Guangdong Woven 20-Nov-18 65 Hangzhou Wonder Garment Ltd. Floor 2, Building 2, No. 10 Kanghui Road, Gongshu District, Hangzhou, Zhejiang Woven 25-Jul-18 136 Haolida fashion co.ltd Group 1,3 Sunliqiao Village, Xingdong Town, Tongzhou District, Nantong, Jiangsu Woven 26-Sep-18 203 Ivy Fashion Co. Ltd. No. 38 Sjjin Road, Yuanshanbei Village, Changping Town, Dongguan, Guang Dong Province, China Knit 15-Aug-18 244 Jiangsu Sunshine Garment 18th Taoxin RD, Xinqiao Town, Jiangyin City, Jiangsu Woven 7-Nov-18 526 Jiaxing Datang Garment Ltd Yuxin Town Industrial Park, Nanhu District, Jiaxing Cut & Sewn 2-May-18 96 Jiayu Fashion Co Ltd (Tongxiang ) Renmin Road No. -

Factory List
*List updated 20th August 2019 FACTORY LIST Country Road Group is committed to driving positive social and environmental change in our supply chain. We mandate safe, inclusive and respectful workplaces wherever our products are manufactured, and are committed to greater transparency of our manufacturing operations. In line with this commitment, Country Road Group has publicly disclosed this list of factories involved in the manufacture of Country Road Group-branded products. This list includes the names and addresses of factories engaged in the manufacture of our goods. Every factory on this list is independently assessed within a rolling audit cycle, and our team works closely with all suppliers and factories to continuously implement improvements. By releasing this information, we aim to provide customers with greater insights into our supply chain. All factories listed, are correct at the time of publishing, and due to the seasonal nature of the retail industry this list is subject to change. SITE NAME ADDRESS REGION ABRAHAM MOON & SONS LTD NETHERFIELD MILLS, GUISELEY, LEEDS, WEST YORKSHIRE UK ACME INDUSTRIES CO., LTD 99 MOO 4, BANGNA-TRAD KM. 35, BANGPLEE-NOI, BANGBOR, SAMUT PRAKAN THAILAND ANHUI BLOSSOM HARDWARE DANFENG ROAD, SIXIAN ECONOMIC DEVELOPMENT ZONE, SUZHOU, JIANGSU CHINA ANHUI SIYI LEATHER GLOVES CO.,LTD NO. 17, CHAJI ROAD, CHENGDONG ECONOMIC DEVELOPMENT ZONE, JING COUNTY, XUANCHENG, ANHUI CHINA ANHUI TENGYANG CLOTHING CO., LTD. BUILDING 3#, YILITENG INDUSTRIAL PARK, QINGHE ROAD, LUYANG INDUSTRIAL PARK, LUYANG DISTRICT, HEFEI, ANHUI CHINA ANKIT BEAD MFG. CO. 13, SHIVAJI NAGAR, MAHMOORGANJ, VARANASI , UTTAR PRADESH INDIA ANM INTERNATIONAL PLOT NO.-99, SECTOR-6, IMT MANESAR, GURGAON, HARYANA INDIA ANTÓNIO MAGALHÃES PINTO LDA RUA DE STª MARIA, Nº 819, FRENTE A, IDÃES PORTUGAL ANTSIRABE KNITWEAR SA TN 1458-AMBOHIMENA, ANTSIRABE, VAKINANKARATRA MADAGASCAR ART WAY CO., LTD. -

Vertical Facility List
Facility List The Walt Disney Company is committed to fostering safe, inclusive and respectful workplaces wherever Disney-branded products are manufactured. Numerous measures in support of this commitment are in place, including increased transparency. To that end, we have published this list of the roughly 7,600 facilities in over 70 countries that manufacture Disney-branded products sold, distributed or used in our own retail businesses such as The Disney Stores and Theme Parks, as well as those used in our internal operations. Our goal in releasing this information is to foster collaboration with industry peers, governments, non- governmental organizations and others interested in improving working conditions. Under our International Labor Standards (ILS) Program, facilities that manufacture products or components incorporating Disney intellectual properties must be declared to Disney and receive prior authorization to manufacture. The list below includes the names and addresses of facilities disclosed to us by vendors under the requirements of Disney’s ILS Program for our vertical business, which includes our own retail businesses and internal operations. The list does not include the facilities used only by licensees of The Walt Disney Company or its affiliates that source, manufacture and sell consumer products by and through independent entities. Disney’s vertical business comprises a wide range of product categories including apparel, toys, electronics, food, home goods, personal care, books and others. As a result, the number of facilities involved in the production of Disney-branded products may be larger than for companies that operate in only one or a limited number of product categories. In addition, because we require vendors to disclose any facility where Disney intellectual property is present as part of the manufacturing process, the list includes facilities that may extend beyond finished goods manufacturers or final assembly locations. -

Hangzhou Overview
Hangzhou Overview Hangzhou Quick Facts Contents • City Name: Hangzhou (杭州, háng zhōu) 01 Hangzhou Quick Facts • Population: 8.8 million 01 Overview • Location: Southeast China 02 Hangzhou Weather • Features: The representative of water 03 What to See in Hangzhou town culture and scenery 12 How to travel in Hangzhou • Area Code: 0571 13 Recommended Hangzhou Tours • Zip Code: 310000 15 What to Do in Hangzhou …………………………………………………………………………… 18 What to Eat in Hangzhou 22 What to Buy in Hangzhou Overview 24 Solo Adventure in Hangzhou Hangzhou, the capital of Zhejiang Province and one of China's seven ancient 26 Hangzhou Hotels capitals, is a coastal city located roughly 30 Hangzhou Transportation midway, north to south, along China's coastline, and a relatively close neighbor to Hangzhou, which lies 180 kilometers farther to the north. Hangzhou is also situated at the mouth of the Qiantang River, and at the southern tip of the Jing-Hang Canal, aka Grand Canal, which extends all the way to Beijing, and which, having evolved over the centuries, finally reached its full length in CE 609, during the Sui (CE 581-617) Dynasty. Although Hangzhou was originally founded during the Qin (BCE 221-207) Dynasty, it was first put on China's map, as it were, during the Sui Dynasty. Indeed, the city's walls were erected at the same time that the Grand Canal - the longest man-made waterway ever conceived - was under construction. Hangzhou Weather Hangzhou enjoys a subtropical monsoon climate with four distinct seasons, which is warm and humid. The annual average precipitation is 1,500 mm, and the average temperature is 16.2℃. -
Sanctioned Entities Name of Firm & Address Date of Imposition of Sanction Sanction Imposed Grounds China Railway Constructio
Sanctioned Entities Name of Firm & Address Date of Imposition of Sanction Sanction Imposed Grounds China Railway Construction Corporation Limited Procurement Guidelines, (中国铁建股份有限公司)*38 March 4, 2020 - March 3, 2022 Conditional Non-debarment 1.16(a)(ii) No. 40, Fuxing Road, Beijing 100855, China China Railway 23rd Bureau Group Co., Ltd. Procurement Guidelines, (中铁二十三局集团有限公司)*38 March 4, 2020 - March 3, 2022 Conditional Non-debarment 1.16(a)(ii) No. 40, Fuxing Road, Beijing 100855, China China Railway Construction Corporation (International) Limited Procurement Guidelines, March 4, 2020 - March 3, 2022 Conditional Non-debarment (中国铁建国际集团有限公司)*38 1.16(a)(ii) No. 40, Fuxing Road, Beijing 100855, China *38 This sanction is the result of a Settlement Agreement. China Railway Construction Corporation Ltd. (“CRCC”) and its wholly-owned subsidiaries, China Railway 23rd Bureau Group Co., Ltd. (“CR23”) and China Railway Construction Corporation (International) Limited (“CRCC International”), are debarred for 9 months, to be followed by a 24- month period of conditional non-debarment. This period of sanction extends to all affiliates that CRCC, CR23, and/or CRCC International directly or indirectly control, with the exception of China Railway 20th Bureau Group Co. and its controlled affiliates, which are exempted. If, at the end of the period of sanction, CRCC, CR23, CRCC International, and their affiliates have (a) met the corporate compliance conditions to the satisfaction of the Bank’s Integrity Compliance Officer (ICO); (b) fully cooperated with the Bank; and (c) otherwise complied fully with the terms and conditions of the Settlement Agreement, then they will be released from conditional non-debarment. If they do not meet these obligations by the end of the period of sanction, their conditional non-debarment will automatically convert to debarment with conditional release until the obligations are met. -
FIT Document(E:\ĸŒçł„ǧ‚Åł¦Å⁄ºç›‹Ç¤¾Æ›•Å±Žæš‡Å¿Š\Å‹Łæ—'Ç
Wang Yicheng, Yang Yang, Ma Renfeng袁 Wu Dandan, Wang Tengfei This paper analyzes the present situation and spatial distribution of the cultural and creative industries in Zhejiang Province, and identifies the four typical models of the development of Zhejiang Cultural Creative In- dustrial Park, such as 要 site transformation, government-led, school-park cooperation, market- It is suggested that the selection of the cluster development model of Zhejiang's cultural creative industry should be coordinated by the government-led, multi-level and regional focus of the market, focus on cultural excavation, personnel training and other major initiatives , prosperity of Zhejiang province Cultural and creative industries. cultural and creative enterprises; cluster; development model; cultural Creative Industrial Park; Zhejiang The creative industry originated from policy rather than academic research, and the UK Creative Industries Task Force defines it as: From the personal creativity, the skill and the tal- ent, through the intellectual property development and the application, has the creation wealth and the employment potential industry. [1] Cultural and creative industries subsequently devel- oped rapidly in Europe and the United States, with its knowledge agglomeration, high relevance of industry and high value-added characteristics, which became one of the pillar industries of the Western countries. Cultural and creative industries located in the cultural industry value chain upstream, [2] can reorganize the production chain of cultural industries, catalyzing the sec- ond to third industry of Cross-border integration. [3] Zhejiang in Zhejiang Province construction of the outline of cultural and creative industry development plan in Zhejiang Province, the cul- tural and creative industries to promote the economic development strategy of Zhejiang Province, emphasis on the development of industry and potential industries, and expected to form a cultural and creative industrial park, cultural and creative industries cluster area, Cre- ative urban three-level space. -

Assessing Land Ecological Security Based on BP Neural Network: a Case Study of Hangzhou, China
1394 JOURNAL OF COMPUTERS, VOL. 8, NO. 6, JUNE 2013 Assessing Land Ecological Security Based on BP Neural Network: a Case Study of Hangzhou, China Heyuan You College of Business Administration, Zhejiang University of Finance and Economics, Hangzhou, China Email: [email protected] Abstract— Due to the increasing stress on the land ecology, feature of sustainable land use [7]. The indicator systems the land eco-security suffers damage. In this paper, the BP which were applied to assess the sustainable use of land neural network and PSR framework were adopted to resources included some indicators reflected land establish the model for assessment of land eco-security, and eco-security such as soil loss/formation ratio [8], forest an empirical study of assessing land eco-security in Hangzhou was done. The results show that the city center cover [9], population density [10], and species loss [11]. district is at serious land eco-security risk; Xiaoshan district Owing to the defects in the accurate analysis of land and Yuhang district are at high land eco-security risk; and ecology, the assessment of land ecological security others counties (cities) are at low risk or intermediate risk. aroused researchers’ attention [6, 12]. In Hangzhou, although some measures are adopted to Back-Propagation (BP) neural network, a method of control the risk of land eco-security, the economic growth training a multi-layer feed-forward artificial neural still has negative impact on the land ecology. The rapid network with the BP algorithm can approximate any industrialization and urbanization increase the risk of land eco-security.