Evidence Based Radiology V10 F. Sardanelli
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Miljø- Og Fødevareudvalget 2016-17 MOF Alm.Del Endeligt Svar På Spørgsmål 892 Offentligt
Miljø- og Fødevareudvalget 2016-17 MOF Alm.del endeligt svar på spørgsmål 892 Offentligt Til: [email protected] ([email protected]), [email protected] (kloepper@waddensea- secretariat.org), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] ([email protected]), [email protected] -

'Ways of Seeing': the Tasmanian Landscape in Literature
THE TERRITORY OF TRUTH and ‘WAYS OF SEEING’: THE TASMANIAN LANDSCAPE IN LITERATURE ANNA DONALD (19449666) This thesis is presented for the degree of Doctor of Philosophy of The University of Western Australia School of Humanities (English and Cultural Studies) 2013 ii iii ABSTRACT The Territory of Truth examines the ‘need for place’ in humans and the roads by which people travel to find or construct that place, suggesting also what may happen to those who do not find a ‘place’. The novel shares a concern with the function of landscape and place in relation to concepts of identity and belonging: it considers the forces at work upon an individual when they move through differing landscapes and what it might be about those landscapes which attracts or repels. The novel explores interior feelings such as loss, loneliness, and fulfilment, and the ways in which identity is derived from personal, especially familial, relationships Set in Tasmania and Britain, the novel is narrated as a ‘voice play’ in which each character speaks from their ‘way of seeing’, their ‘truth’. This form of narrative was chosen because of the way stories, often those told to us, find a place in our memory: being part of the oral narrative of family, they affect our sense of self and our identity. The Territory of Truth suggests that identity is linked to a sense of self- worth and a belief that one ‘fits’ in to society. The characters demonstrate the ‘four ways of seeing’ as discussed in the exegesis. ‘“Ways of Seeing”: The Tasmanian Landscape in Literature’ considers the way humans identify with ‘place’, drawing on the ideas and theories of critics and commentators such as Edward Relph, Yi-fu Tuan, Roslynn Haynes, Richard Rossiter, Bruce Bennett, and Graham Huggan. -

50Th ANNUAL REPORT 2011-2012
50th ANNUAL REPORT 2011-2012 Abridged format Patron: Sir James D Wolfensohn President: Peter Hasko Vice President: Kimberly Everett/Patrick Regan Hon Secretary: Naomi Flutter Hon Treasurer: Patrick Regan Councillors: Ted Blamey, Richard Broinowski, Lisa George, Charles Graham, Justin Greiner, Chris Smith, Tony Thirlwell Chapter Convenors: Queensland: David Henderson ACT: Michelle Patterson, South Australia: Harley Hooper Western Australia: Ken Perry Email: [email protected]. Website: www.harvardclub.org.au Note: A full copy of the Report of Factual Findings can be viewed or downloaded from the Members Only section of the website. A user name and password will be issued by the Administrator upon receipt of an application by email. Page 1 of 16 THIS IS A BLANK PAGE Page 2 of 16 NOTICE OF ANNUAL GENERAL MEETING The 50th Annual General Meeting of the Harvard Club of Australia will be held on Thursday 20 September 2012 at the Waterfront Restaurant, The Rocks, Sydney commencing at 6.30 pm. Agenda 1. Confirmation of Minutes 49th Annual General Meeting of the Harvard Club of Australia was held on Thursday, 10 November 2011 at QVB Tea Room, 455 George Street Sydney 2. President’s Report 3. Approval of Annual Financial Statements Approval of the Financial Statements for the Club for the year ended 31 December 2011. 4. Election of Auditors KPMG retire at the meeting and being eligible seek to be reappointed to conduct the Report of Factual Findings 5. Election of Executive Office Bearers The Constitution provides that Executive Office Bearers retire at each Annual General Meeting (AGM) and can only hold that same office for two years. -

The Racgp John Murtagh Library
THE RACGP JOHN MURTAGH LIBRARY RESEARCH HARD COPY ITEMS STOCKED As of: 14/01/2020 The Royal Australian College of General Practitioners (RACGP) 100 Wellington Parade East Melbourne Vic 3002 Tel: +61 (3) 8699 0519 Fax: +61 (3) 8699 0400 Email: [email protected] www: https://www.racgp.org.au/clinical-resources/john- murtagh-library RACGP John Murtagh Catalogue: Research Adequacy of sample size in health studies / edited by Stephen K. Lwanga ; with contributions by Stanley Lemeshow, David W Hosmer, Jr, Janelle Klar ; with editorial assistance by James L Duppenthaler. Chichester : Wiley, for the World Health Organization, c1990. SAMPLING STUDIES RESEARCH DESIGN Lwanga, Stephen K. World Health Organization. 519.52 ADE An anthology of literature reviews by GPEP researchers. Volume 2 / Anne Magarey, Wendy Rogers, Bronwyn Veale. [Adelaide] : National Information Service, Dept. of General Practice, Flinders Medical Centre, 2000 PHYSICIANS, FAMILY FAMILY PRACTICE RESEARCH PROGRAM EVALUATION REVIEW LITERATURE General Practice Evaluation Program. National Information Service. Flinders Medical Centre. Dept. of General Practice. 610.6950994 MAG Basic & clinical biostatistics [electronic resource] / Beth Dawson, Robert G. Trapp. 4th ed. New York : McGraw-Hill. BIOMETRY Biometry - Computer network resources. Trapp, Robert G The Cochrane Library [electronic resource]. St. Leonards, N.S.W. : Health Communication Network. RESEARCH DESIGN CLINICAL TRIALS META-ANALYSIS REVIEW LITERATURE EVIDENCE-BASED MEDICINE Randomized Controlled Trials. Cochrane Collaboration. 618.32 COC Page 1 RACGP John Murtagh Catalogue: Research 3/07/19 Conducting research in the practice setting / edited by Martin J. Bass ... et al. Newbury Park, Ca. : Sage Publications, c1993. FAMILY PRACTICE RESEARCH DESIGN Bass, Martin J. 610.72 CON Designing clinical research : an epidemiologic approach / edited by Sephen B. -
9781920898359 Appendices R
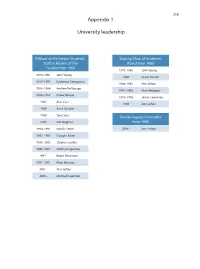
319 Appendix 1 University leadership Fellows of the Senate Students, Deputy Chair of Academic Staff or Alumni of the Board from 1980 Faculty from 1980 1978–1980 John Young 1978–1981 John Young 1986 Susan Dorsch 1979–1993 Katherine Georgouras 1986–1987 Ann Sefton 1983–1986 Andrew Refshauge 1991–1993 Alan Pettigrew 1986–1987 Diana Temple 1992–1996 James Lawrence 1987 Alan Cass 1996 Ann Sefton 1989 Anna Donald 1989 Tony Sara Senate Deputy Chancellor 1989 Eric Wegman from 1980 1990–1991 Natalie Smith 2004– Ann Sefton 1993–1995 Douglas Baird 1995–2002 Stephen Leeder 1996–2001 Michael Copeman 1997– Robin Fitzsimons 1997–2001 Peter Burrows 2001– Ann Sefton 2005– Michael Copeman 320 Appendix 2 Deans of the Faculty of Medicine from 1980 1974 –1989 Richard S Gye 1989 –1996 John Atherton Young 1997–2002 Stephen Leeder 2003 – Andrew Coats Appendix 3 Current Emeritus Professors Barry Baker John Little Tony Basten William McCarthy Geoffrey Berry James McLeod Charles Blackburn Russell Meares Charles Bridges-Webb Gerald Milton Neil Buchanan Kim Oates Peter Castaldi Murray Pheils John Chalmers Wai-On Phoon Patrick De Burgh Thomas Reeve Susan Dorsch Douglas Saunders Hans Freeman Ann Sefton Kerry Goulston Ainslie Sheil Richard Gye Frederick Stephens Akos Gyory Michael Taylor Noel Hush Thomas Taylor Gordon Johnson Stewart Truswell Charles Kerr John Turtle James Lawrence Robert Wake 321 Appendix 4 Professors appointed or promoted 1980–2005 1980 W Cramond 1989 Nicholas Hunt 1994 Leigh Delbridge 1980 Graeme Johnston 1989 John Sutton 1994 Ian Frazer 1980 Philip -

HARC E-Bulletin Issue 8
HARC eBulletin Quarterly e-Bulletin of the Hospital Alliance for Research Collaboration Issue 8 Welcome to our latest HARC eBulletin. In this eBulletin we highlight recent groundbreaking March reviews, research and reports that are relevant for Australian health policy deliberations. In 2009 this edition we report on new evidence for: acute geriatric units; a surgical checklist that has been shown to reduce deaths and complications that is being implemented across the NHS; Print Version processes to improve post-acute care such as discharge planning and telephone follow-up care. On to a sombre note, the news of Dr Anna Donald’s untimely passing was received by the Australian and international research, policy and clinical communities with great sadness. In our news section below we profile her obituaries in the British Medical Journal (by Richard Smith and Muir Gray) and Sydney Morning Herald that commend her unwavering dedication to improving health services by the application of best available evidence – a goal that resonates with HARC. We pass on our condolences to Anna’s family, friends and colleagues. Mary Haines, Health Services Research Director News NSW successful in latest round of NHMRC capacity building grants for population health and health services research This eBulletin is NSW has won five of nine capacity building grants totaling $18.2million. The successful grants produced by the include “OSPREY: Building capacity for research to improve health services for mothers, HARC Office at babies and children”, which brings together a team of investigators from the University of the Sax Institute. Western Sydney, the University of Sydney, the University of Western Australia and the Sax Institute. -

Clinical Governance and the Drive for Quality Improvement in the New NHS in England Gabriel Scally, Liam J Donaldson
The NHS’s 50th anniversary sugar and adjust their insulin doses, achieving far bet- 2023 seek the continual improvement of an NHS full ter control than when the doctor was making the insu- of knowledge, taking the best as its norm, growing its lin adjustments.9 You learned from Dr David Sobel at capacity as a full and integrated system of shared effort, Kaiser Permanente in America, who trained chroni- wasting little, and respecting every patient as an cally ill adults to provide care and education to other individual. You continue to know that you started off chronically ill adults, achieving better health status out- right in 1948, and with some important midcourse 10 comes and lower cost for both teachers and students. corrections, you remain well on track. Maybe some day You built your programmes on evidence of the benefits healthcare leaders in the United States will catch up. 11 of patient self care in studies of asthma treatment, I am sure you will help them if they ask. hypertension treatment, and self diagnosis of urinary 12 tract infection. The author thanks Paul Plsek, John Oldham, Diane Plamping, Jo By the early 21st century, the NHS was becoming a Bufford, and Jan Filotowski for helpful comments. truly patient centered clinical care system. The empha- sis today is on helping people with acute and chronic illnesses to become experts in their own care whenever 1 Secretary of State for Health. The new NHS. London: Stationery Office, 1997. (Cm 3807.) they wish, able to participate fully in their own diagno- 2 Rogers E. -

Proposed Format for Abridged Annual Report
5 2 n d ANNUAL REPORT 2013- 2 0 1 4 Abridged format Patron: Sir James D Wolfensohn President: Patrick Regan Vice President: Naomi Flutter Hon Secretary: Lisa George Hon Treasurer: Justin Greiner Councillors: Scoti Albrecht, Ted Blamey, Richard Broinowski, Charles Graham, Joanna Marsh, Leila Morsy, Aaron Patrick, Chris Smith, Tony Thirlwell Chapter Convenors: Queensland: David Henderson ACT: Lucinda Glover and Jamie Snashall South Australia: Harley Hooper Western Australia: Elizabeth Carr and Mark Paganin Email: [email protected]. Website: www.harvardclub.org.au This Page is Blank NOTICE OF ANNUAL GENERAL MEETING The 51st Annual General Meeting of the Harvard Club of Australia will be held on Thursday October 16 2014 at Level 16, 126 Philip Street Sydney, commencing at 6.30 pm. Agenda 1. Confirmation of Minutes 51st Annual General Meeting of the Harvard Club of Australia was held on Thursday, 26 September 2013 at the Union Universities and Schools Club, Sydney. 2. President’s Report 3. Approval of Annual Financial Statements Approval of the Financial Statements for the Club for the year ended 31 December 2013. 4. Election of Auditors The HCA’s Group Auditors is Quest Chartered Accountants we offer to reappoint them to act again as auditors in the next Financial Year 5. Constitutional amendment Proposal to amend Clause V of the constitution to add, at the end of the clause: The office of President may be occupied by two individuals who will, in such circumstance, both serve as President for the term of their appointment. Consistent with Clause XXIII, this amendment must be notified to members not less than 21 days before the meeting and must be sanctioned by two-thirds of members present and voting in person, subject to at least 20 members voting on the occasion. -

The Scottish Parliament Cross-Party Group for the Prevention and Healing of Adverse Childhood Experiences (Aces)
The Scottish Parliament Cross-Party Group for the Prevention and Healing of Adverse Childhood Experiences (ACEs) Committee Room 2, Scottish Parliament, 27th November 2019, 6-8 pm Welcomes and introductions MSPs who attended: • Gail Ross, SNP MSP for Caithness, Sutherland and Ross • Rona Mackay, SNP MSP for Strathkelvin & Bearsden • Jenny Gilruth, SNP MSP for Mid Fife & Glenrothes Other attendees: • Mark Ballard, Children 1st • Superintendent Ann Bell, Police Scotland • Dr Alana David, British Psychological Society and NHS Lothian • Anna Donald, Head of Victims and Witnesses Unit, Scottish Government • James Docherty, Violence Reduction Unit • George Hosking, WAVE Trust • Tracey Jenkins, GCVS • Anthoulla Koutsoudi, 70/30 Campaign and WAVE Trust • Ryan McShane MSYP, Scottish Youth Parliament/Who Cares? Scotland • Dr Claire Ogilvie, British Psychological Society and NHS Greater Glasgow and Clyde • Janine Rennie, Wellbeing Scotland • Louise Slorance, RCPCH Scotland Apologies: • Liam McArthur, Liam Kerr, Anne McDonald, Nicola Wylie, David Mitchell, Debbie McCall Minutes of 29th May meeting: The Minutes of the meeting were agreed. Matters arising 1. Maree Todd, the Minister for Children and Young People, has replied to the letter sent to her. 2. We are awaiting further information from the Minister for Mental Health on how the additional £32m for mental health will be spent. Guest Speaker Presentations: A Trauma Informed Justice Service CPG NOV 2019 – Summary of presentation by Anna Donald, Head of Victims and Witnesses Unit, Scottish Government Criminal Justice Department of SG has been looking at a trauma-informed workforce. However, there are wider issues of trauma in prisoners, offenders, victims and witnesses. The Scottish Government has a four-step approach: 1 1. -

Great Scot!: the Scottish Plan to End Homelessness and Lessons for the Housing Rights Movement in the United States
Georgetown Journal on Poverty Law & Policy Volume XVI, Number 1, Winter 2009 Great Scot!: The Scottish Plan to End Homelessness and Lessons for the Housing Rights Movement in the United States Eric S. Tars and Caitlin Egleson* Scotland has a great mission to end homelessness by 2012. We share a similar vision and we base it on the notion of abolishing a social wrong, a social evil. If you look at the initiatives in Scotland and in the United States, there are great similarities, such as on the priority of partnership and increased resources from central government. The strategies that are being developed in Scotland are aimed to accomplish certain results within a specific time frame, moving away from managing homelessness to ending it. What we’ve come to realize in the last 20 years is that no one level of government and no one element of the private sector can do this alone. This is a national problem with local solutions. —Philip Mangano, Chair of the Inter-Agency Council on Homelessness1 INTRODUCTION An estimated 3.5 million people experience homelessness in the United States in any given year, including over 1 million children.2 While many face literal street homelessness, millions more are doubled up with friends or family, or living paycheck-to-paycheck and in danger of losing their housing at any point.3 At the core of this problem is an overwhelming deficit of affordable housing, with a full-time minimum wage worker unable to afford a single bedroom apartment anywhere in the country.4 The current foreclosure crisis is making matters worse, both for homeowners who are losing their life’s savings and being forced into * Eric S. -

Evidence Based Medicine: an Approach to Clinical Problem-Solving
repression inflicted by the previous regime, or perhaps 2 Benatar SR. Medicine and health care in South Africa-five years later. NEngIlMed 1991;325:30-6. orchestrated by radical union leaders. Whatever the 3 Van Rensburg HCJ, Benatar SR The legacy of apartheid in health and health explanation, they show that the maturity displayed by care. SouthAfricanJoumal ofSociology 1993;24:99-1 11. the South Africans at the time of the elections has 4 Department of National Health and Population Development. Health trends in South Africa 1993. Pretoria: The Department, 1994:19. not been shown by its striking workers. The honey- 5 Blumson D. The pathology of poverty. In: Huddle K, Dubb A, eds. moon phase between South African politicians and Baragwanath Hospital, 50 years: a medical miscellany. Bertscham South Africa: Baragwanath Hospital, 1994:7-11. their electorate is becoming tumultuous and the honey- 6 World Bank. World development report 1993. Investing in heakh. Oxford: Oxford moon is being threatened. University Press, 1993:238-9. 7 Vital signs. Asiaweek. 1994 December 7:47-88. 1 Benatar SR. Medicine and health care in South Africa. N Engl J Med 8 Van der Linde 1. Strike at King Edward: important lessons. South African 1986;315:527-32. MedicalJournal 1994;84:15. Evidence based medicine: an approach to clinical problem-solving William Rosenberg, Anna Donald _ Doctors within the NHS are confronting major electronic databases and widespread computer literacy changes at work. While we endeavour to improve the now give doctors access to enormous amounts of data. quality of health care, junior doctors' hours have Evidence based medicine is about asking questions, been reduced and the emphasis on continuing finding and appraising the relevant data, and harness- medical education has increased. -

55Th ANNUAL REPORT 2016 HARVARD CLUB of AUSTRALIA
HARVARD CLUB OF AUSTRALIA 55th ANNUAL REPORT 2016 HARVARD CLUB OF AUSTRALIA INC and its subsidiary HCA PHILANTHROPY PTY LTD Patron – Sir James D Wolfensohn MBA’59 President – Justin Greiner MBA’00 Vice-President – Charles Graham MBA’01 Treasurer – Joanna Marsh AB’03 Secretary – Ricky Campbell-Allen EDM‘10 Councillors Ted Blamey MBA’70, Elizabeth Carr MPA’04, Kimberly Everett MUP’98, Naomi Flutter MPP’98, Peter Hasko PMD’93, Jon Lindsay MBA’87, Tempe MacGowan MLAUD‘93, Susan Overall AB‘14, Aaron Patrick MPP’04, Patrick Regan EDM’06, Kirthana Sharma MPH’00, Chris Smith MBA’77, Jamie Snashall MPA’08, Luke Woodward MPA’97 and Yu Zhang LLM’10. Chapter Convenors Queensland – Jon Lindsay MBA’87 ACT – Jamie Snashall MPA’08 Western Australia – Elizabeth Carr MPA’04 HCA Philanthropy Pty Ltd Directors Christopher Smith MBA‘77 (Chairman), Melinda Muth MBA’81 (Secretary and Director), Naomi Flutter MPP’98 (Resigned 31 October 2016), Charles Graham MBA’01, Jon Chambers OPM’91, Kimberly Everett MUP’98, Joanna Marsh AB’03 (Appointed 31 October 2016) and Justin Greiner MBA’00 (Appointed 31 October 2016) Email – [email protected] Website – harvardclub.org.au Harvard Club of Australia NOTICE OF ANNUAL GENERAL MEETING The 55th Annual General Meeting of the Harvard Club of Australia will be held on Thursday 29 June 2017 at JBWere, Level 42, Governor Phillip Tower, 1 Farrer Place, Sydney NSW commencing at 6.00pm. Agenda 1. Attendance and apologies 2. Confirmation of minutes of previous Annual General Meeting 54th Annual General Meeting of the Harvard Club of Australia was held on Wednesday 7 September 2016 at Deutsche Bank, Level 16, 126 Phillip Street, Sydney NSW commencing at 6.30pm.