Colorimetric Determination of Blood Sugar Level” ______
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Your Blood Test Lab Results
Understanding Your Blood Test Lab Results A comprehensive "Health Panel" has been designed specifically to screen for general abnormalities in the blood. This panel includes: General Chemistry Screen or (SMAC), Complete Blood Count or (CBC), and Lipid examination. A 12 hour fast from all food and drink (water is allowed) is required to facilitate accurate results for some of the tests in this panel. Below, is a breakdown of all the components and a brief explanation of each test. Abnormal results do not necessarily indicate the presence of disease. However, it is very important that these results are interpreted by your doctor so that he/she can accurately interpret the findings in conjunction with your medical history and order any follow-up testing if needed. The Bernards Township Health Department and the testing laboratory cannot interpret these results for you. You must speak to your doctor! 262 South Finley Avenue Basking Ridge, NJ 07920 www.bernardshealth.org Phone: 908-204-2520 Fax: 908-204-3075 1 Chemistry Screen Components Albumin: A major protein of the blood, albumin plays an important role in maintaining the osmotic pressure spleen or water in the blood vessels. It is made in the liver and is an indicator of liver disease and nutritional status. A/G Ratio: A calculated ratio of the levels of Albumin and Globulin, 2 serum proteins. Low A/G ratios can be associated with certain liver diseases, kidney disease, myeloma and other disorders. ALT: Also know as SGPT, ALT is an enzyme produced by the liver and is useful in detecting liver disorders. -

Ionized Calcium: Analytical Challenges and Clinical Relevance
16 Review Article Page 1 of 16 Ionized calcium: analytical challenges and clinical relevance Aghilès Hamroun1, Jean-David Pekar2, Arnaud Lionet1, Amjad Ghulam3, Patrice Maboudou2, Ambroise Mercier2, Thierry Brousseau2, Guillaume Grzych3,4, François Glowacki1,5 1CHU Lille, Service de Néphrologie, F-59000, Lille, France; 2CHU Lille, Biochimie Automatisée (UF-8832), Pôle de Biologie Pathologie Génétique, F-59000, Lille, France; 3CHU Lille, Service « Hormonologie, Métabolisme, Nutrition, Oncologie », Pôle de Biologie Pathologie Génétique, Lille, France; 4INSERM, U1011-EGID, Institut Pasteur de Lille, F-59000, Lille, France; 5EA 4483-IMPECS-IMPact of Environmental Chemicals on Human Health, Research Department, 59045, Lille, France Contributions: (I) Conception and design: G Grzych, JD Pekar, A Hamroun; (II) Administrative support: G Grzych, JD Pekar, A Hamroun; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: G Grzych, JD Pekar, A Hamroun; (V) Data analysis and interpretation: G Grzych, JD Pekar, A Hamroun; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Dr. Guillaume Grzych, MD, MSc. Laboratory of Biochemistry, Biology and Pathology Center, Lille University Hospital, Pr J. Leclercq Boulevard, 59037 Lille, France. Email: [email protected]. Abstract: Calcium is a central electrolyte involved in many physiological functions commonly measured in clinical practice. Abnormal calcium levels are frequently found, and could have serious clinical consequences. The free form of calcium, also called ionized calcium, plays a determining role in the human body. Its measurement still remains limited by technical, analytical and financial constraints. To overcome these limits, many formulas have been developed to estimate the ionized calcium value. -

Medical Tests
Waldenstrom’s Macroglobulinemia Medical Tests WALDENSTROM’S MACROGLOBULINEMIA MEDICAL TESTS The IWMF Vision Statement A World Without WM (Waldenstrom's macroglobulinemia). The IWMF Mission Statement Support and educate everyone affected by Waldenstrom's macroglobulinemia (WM) while advancing the search for a cure. To accomplish this vision, the IWMF offers WM patients, caregivers, family members, and friends six invaluable services: • Information from our website and our publications written in a patient-friendly way to promote understanding of our rare disease • Education at our annual Educational Forum to help patients and caregivers learn about our disease from WM researchers and clinicians • On-going updates about WM and the IWMF sent through our quarterly IWMF Torch magazine and our NEWS releases • Peer support from others who’ve been where you are • Information for medical professionals who may have limited experience with our rare disease • Research directed to better treatments while we search for a cure Since WM is a rare disease, the IWMF relies upon individuals for financial support and upon volunteers to do much of its work. Published by the International Waldenstrom’s Macroglobulinemia Foundation (IWMF) This information has been provided by the IWMF at no cost to you. Please consider Joining and/or contributing to the IWMF to enable us to continue to provide materials like this to support research toward better treatments and a cure for Waldenstrom’s macroglobulinemia. You may Join and/or contribute at our website, www.iwmf.com, or you may mail your contribution to: 6144 Clark Center Avenue, Sarasota, FL 34238. IWMF is a 501(c)(3) Tax Exempt Non-Profit Organization, Fed ID #54-1784426. -

Lipase Interpretive Summary
Lipase Interpretive Summary Description: Lipase is an enzyme produced primarily in the pancreas to break down dietary fats in the small intestine. Decreased Lipase Common Causes Artifact o Hemolysis directly inhibits lipase activity in the assay Decreased lipase is not clinically significant. Increased Lipase Common Causes Pancreatic acinar cell damage o Inflammation o Necrosis o Neoplasia o Pancreatic duct obstruction Gastrointestinal inflammation Decreased renal clearance/inactivation (any condition that reduces glomerular filtration rate [GFR]) o Pre-renal conditions . Shock . Dehydration . Poor cardiac output o Renal disease o Post-renal disorders . Urinary tract obstruction or rupture Glucocorticoid treatment Uncommon Causes Hepatic neoplasia (dogs) Peritonitis Bowel obstruction Widespread steatitis Related Findings Pancreatic acinar cell damage o Increased amylase o Increased Spec cPL®/Spec fPL®. o Increased ALP +/- bilirubin o Increased glucose o Inflammatory leukogram on CBC Gastrointestinal inflammation o Increased BUN with gastric bleeding Decreased renal clearance/inactivation o Pre-renal . Increased hematocrit and total protein Generated by VetConnect® PLUS: Lipase Page 1 of 2 . Increased BUN, creatinine, and possibly phosphorous . Increased urine specific gravity . Decreased blood pressure (shock, poor cardiac output) o Renal . Increased BUN, creatinine, phosphorus . Isosthenuria o Post-renal . Increased BUN, creatinine, and possibly phosphorus . Abnormalities on urinalysis (blood, inflammation, crystals, etc.) . Abnormalities associated with urinary tract obstruction/rupture on abdominal ultrasound or contrast studies Additional Information Physiology Lipase is a cytoplasmic enzyme produced by the pancreas and released into the small intestine to catalyze the hydrolysis of triglycerides for absorption into the lacteals and gastrointestinal blood vessels. In addition to pancreatic acinar cells, lipase is produced by gastric mucosal cells, hepatocytes, and many extrahepatic cells (including adipocytes and myocytes). -

T. Serum Total Protein Laboratory Procedure Manual
Laboratory Procedure Manual Analyte: Total Protein Matrix: Serum Method: Roche Cobas 6000 (c501 module) Method No.: Revised: As performed by: University of Minnesota Advanced Research and Diagnostic Laboratory (ARDL) 1200 Washington Ave S, Suite 175 Minneapolis, MN 55415 Contact: Anthony Killeen, MD, PhD, Laboratory Director Jennifer Peters, MT, ASCP, Laboratory Manager Important Information for Users The Advanced Research and Diagnostic Laboratory (ARDL) periodically refine these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. Total Protein NHANES 2017-2018 Public Release Data Set Information This document details the Lab Protocol for testing the items listed in the following table: Data File Name Variable Name SAS Label BIOPRO_J LBXSTP Total Protein (g/dL) 1 of 14 Total Protein NHANES 2017-2018 1. SUMMARY OF TEST PRINCIPLE AND CLINICAL RELEVANCE Plasma proteins are synthesized primarily in the liver, but also in plasma cells, lymph nodes, the spleen and bone marrow. Disease processes will affect the overall concentration of protein, as well the ratio of the protein fractions that comprise the total amount. Hypoproteinemia can be caused by loss of blood, sprue, nephrotic syndrome, severe burns, salt retention syndrome and insufficient dietary protein intake (Kwashiorkor). Hyperproteinemia may be observed in acute dehydration and illnesses like multiple myeloma. Changes in the relative amounts of the protein fractions can occur without a change in the total protein. The albumin/globulin ratio is commonly used to screen for abnormal protein fraction concentrations. -

Serology and Other Adaptive Immune Response Testing for COVID-19
Factsheet Serology and Other Adaptive Immune Response Testing for COVID-19 Rachel West, PhD April 27, 2021 Background Serology and other adaptive immune response tests can be used to determine a person’s exposure and immunity to specific pathogens. Serology tests analyze serum—a component of whole blood— to identify whether a person has developed an immune response by measuring antibodies or antigens. In contrast, other adaptive immune response tests analyze whole blood to measure, for example, the activity of immune cells, such as T cells. Antibodies are produced specific to antigens, which are particles of a pathogen that are perceived as foreign by the immune system. Serology tests are often used in viral infections to see if the patient has developed an immune response to a pathogen of interest, such as influenza. These tests cannot be used to diagnose infection or to ensure protective immunity. Serology and other adaptive immune response tests can only be used to understand the presence of an immune response to a certain antigen. There are several types of serology tests.1 1. Neutralization tests can indicate whether the patient has active, functional antibodies to the pathogen in question by measuring how much the antibodies can inhibit viral growth in the lab (Figure 1). This can be used with SARS-CoV-2 virus in a biosafety level 3 setting or with pseudoviruses that express certain SARS-CoV-2 proteins in a lower biosafety level setting.2 Surrogate neutralization assays may use a modified ELISA technique (see below) to establish levels of neutralizing antibodies.3 2. -

Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications
proteomes Article Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications Josephine N. Henok 1, Benjamin I. Okeleye 2,*, Elizabeth I. Omodanisi 2 , Seteno K. O. Ntwampe 2,3 and Yapo G. Aboua 1,2 1 Department of Health Sciences, Faculty of Health and Applied Sciences, Namibia University of Science and Technology (NUST), Private Bag 13388, Windhoek, Namibia; [email protected] (J.N.H.); [email protected] (Y.G.A.) 2 Bioresource Engineering Research Group (BioERG), Department of Biotechnology, Faculty of Applied Sciences, Cape Peninsula University of Technology, P.O. Box 652, Cape Town 8000, South Africa; [email protected] (E.I.O.); [email protected] (S.K.O.N.) 3 School of Chemical and Minerals Engineering, North-West University, Private Bag X1290, Potchefstroom 2520, South Africa * Correspondence: [email protected]; Tel.: +27-214603981 Received: 14 February 2020; Accepted: 30 March 2020; Published: 15 April 2020 Abstract: A reference range is an essential part of clinical laboratory test interpretation and patient care. The levels of total serum protein (TSP) are measured in sera to assess nutritional, liver, and kidney disorders. This study determined the TSP reference range with respect to gender, age, and region in Namibia. A retrospective cross-sectional study was conducted to determine the TSP reference range among 78,477 healthy participants within the ages of less than one year to more than 65 years in 14 regions of Namibia. The reference range of TSP was 51–91 g/L for females and 51–92 g/L for males. A reduced TSP range of 48.00–85.55 g/L (2.5–97.5 percentiles) was established at <1–5 years and increased towards adolescence. -

Acute Pancreatitis with Normal Serum Lipase: a Case Series
JOP. J Pancreas (Online) 2010 Jul 5; 11(4):369-372. CASE SERIES Acute Pancreatitis with Normal Serum Lipase: A Case Series Anish M Shah1, Rodney Eddi2,3, Shivangi T Kothari2,3, Charbel Maksoud2,3, William Scott DiGiacomo3, Walid Baddoura2,3 1Department of Internal Medicine and 2Division of Gastroenterology, Saint Joseph’s Regional Medical Center. Paterson, NJ, USA. 3Seton Hall University School of Health and Medical Sciences. South Orange, NJ, USA ABSTRACT Context Acute pancreatitis is diagnosed on the basis of clinical features, biochemical tests and imaging studies. Normal serum amylase level has been reported in the setting of acute pancreatitis but normal serum lipase level in acute pancreatitis is extremely rare. Case report Herein, we present a case series of acute pancreatitis with normal serum lipase levels along with a review of the topic. Conclusion In appropriate clinical setting, the diagnosis of acute pancreatitis should be entertained even with normal serum amylase and lipase levels. INTRODUCTION 3) computed tomography (CT) scan findings compatible with acute pancreatitis [4]. Serum amylase Acute pancreatitis is one of the most common causes and lipase levels threefold or more than normal are for hospitalization in the United States, accounting for seen in acute pancreatitis and, in the appropriate around 220,000 cases per year [1]. Among the new clinical setting, used for diagnosis [5]. Normal serum cases, 80% are interstitial and 20% are necrotizing. amylase levels have been reported in some cases of Acute pancreatitis carries an overall mortality of acute pancreatitis, but serum lipase levels are usually around 5% and as high as 47% in patients with multi- elevated [6]. -

Normal Fasting Plasma Glucose Levels and Type 2 Diabetes in Young Men
The new england journal of medicine original article Normal Fasting Plasma Glucose Levels and Type 2 Diabetes in Young Men Amir Tirosh, M.D., Ph.D., Iris Shai, R.D., Ph.D., Dorit Tekes-Manova, M.D., Eran Israeli, M.D., David Pereg, M.D., Tzippora Shochat, M.Sc., Ilan Kochba, M.D., and Assaf Rudich, M.D., Ph.D., for the Israeli Diabetes Research Group abstract background From the Medical Corps Headquarters The normal fasting plasma glucose level was recently defined as less than 100 mg per (A.T., E.I., T.S., I.K.) and the Center for deciliter (5.55 mmol per liter). Whether higher fasting plasma glucose levels within this Medical Services (D.T.-M.), Israel Defense Forces Medical Corps; the Department of range independently predict type 2 diabetes in young adults is unclear. Internal Medicine A, Sheba Medical Cen- ter, Tel-Hashomer (A.T.); the S. Daniel Abra- methods ham International Center for Health and Nutrition (I.S., A.R.), the Department of We obtained blood measurements, data from physical examinations, and medical and Epidemiology (I.S.), and the Department lifestyle information from men in the Israel Defense Forces who were 26 to 45 years of Clinical Biochemistry (A.R.), Faculty of of age. Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva; and the Depart- ment of Internal Medicine A, Meir Hospital, results Sapir Medical Center, Kfar-Sava (D.P.) — A total of 208 incident cases of type 2 diabetes occurred during 74,309 person-years of all in Israel. Address reprint requests to Dr. -

Serology Testing and COVID-19 – What We Know So Far
SYNOPSIS 06/07/2020 Serology testing and COVID-19 – What We Know So Far Introduction PHO is actively monitoring, reviewing and assessing relevant information related to Coronavirus Disease 2019 (COVID-19) and the virus that causes it, SARS-CoV-2. “What We Know So Far” documents are intended to provide a rapid review of the evidence related to a specific aspect or emerging issue related to COVID-19. The development of these documents includes a systematic search of the published literature as well as scientific grey literature (e.g., ProMED, CIDRAP, Johns Hopkins Situation Reports) and media reports, where appropriate. Relevant results are reviewed and data extracted for synthesis. All “What We Know So Far” documents are reviewed by PHO subject-matter experts before posting. As the COVID-19 outbreak continues to evolve and the scientific evidence rapidly expands, the information provided in these documents is only current as of the date of posting. Key Points Serological assays detect the host antibody response to COVID-19. Tests have been developed to detect immunoglobulin A (IgA), IgM, IgG, and total antibody. Independent evaluations of the various COVID-19 serological assays have shown highly variable performance, with sensitivities and specificities ranging from 0%-100%. Correlation between COVID-19 serological assays (detecting antibodies) and neutralization assays (measuring functional neutralizing antibodies) varies, based on a limited number of studies. It is currently not known whether a COVID-19-specific antibody response correlates with immunity and if so, whether serological assays can adequately measure immunity. At this time, serological assays can only determine past exposure to COVID-19. -
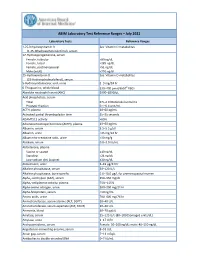
ABIM Laboratory Test Reference Ranges ̶ July 2021
ABIM Laboratory Test Reference Ranges ̶ July 2021 Laboratory Tests Reference Ranges 1,25-Dihydroxyvitamin D See Vitamin D metabolites (1,25-dihydroxycholecalciferol), serum 17-Hydroxyprogesterone, serum Female, follicular ˂80 ng/dL Female, luteal ˂285 ng/dL Female, postmenopausal ˂51 ng/dL Male (adult) ˂220 ng/dL 25-Hydroxyvitamin D See Vitamin D metabolites (25-Hydroxycholecalciferol), serum 5-Hydroxyindoleacetic acid, urine 2–9 mg/24 hr 6-Thioguanine, whole blood 230–400 pmol/8x108 RBCs Absolute neutrophil count (ANC) 2000–8250/μL Acid phosphatase, serum Total 0.5–2.0 (Bodansky) units/mL Prostatic fraction 0.1–0.4 unit/mL ACTH, plasma 10–60 pg/mL Activated partial thromboplastin time 25–35 seconds ADAMTS13 activity >60% Adrenocorticotropic hormone (ACTH), plasma 10–60 pg/mL Albumin, serum 3.5–5.5 g/dL Albumin, urine ˂25 mg/24 hr Albumin-to-creatinine ratio, urine ˂30 mg/g Aldolase, serum 0.8–3.0 IU/mL Aldosterone, plasma Supine or seated ≤10 ng/dL Standing ˂21 ng/dL Low-sodium diet (supine) ≤30 ng/dL Aldosterone, urine 5–19 μg/24 hr Alkaline phosphatase, serum 30–120 U/L Alkaline phosphatase, bone specific 5.6–18.0 µg/L for premenopausal women Alpha1-antitrypsin (AAT), serum 150–350 mg/dL Alpha2-antiplasmin activity, plasma 75%–115% Alpha-amino nitrogen, urine 100–290 mg/24 hr Alpha-fetoprotein, serum ˂10 ng/mL Amino acids, urine 200–400 mg/24 hr Aminotransferase, serum alanine (ALT, SGPT) 10–40 U/L Aminotransferase, serum aspartate (AST, SGOT) 10–40 U/L Ammonia, blood 40–70 μg/dL Amylase, serum 25–125 U/L (80–180 [Somogyi] units/dL) -

UNDERSTANDING YOUR MULTIPLE MYELOMA LAB TESTS This Lab Tracker Booklet Explains Common Tests for Patients with Multiple Myeloma
UNDERSTANDING YOUR MULTIPLE MYELOMA LAB TESTS This lab tracker booklet explains common tests for patients with multiple myeloma. If you have any questions about your test results, discuss them with your healthcare team. LAB TEST TRACKER This worksheet can serve as a personal record of your lab test results. Reference ranges— values that are considered normal in healthy individuals—are provided below as guides. Note that ranges vary among laboratories. Date of laboratory test Mark the test values in the Reference ranges column below each date COMPLETE BLOOD COUNT (CBC)1,2 Pages 4-5 White blood cells (WBCs) 3.5-10.5 2 109/L Neutrophils 1.56-6.45 2 109/L Males: 4.32-5.72 2 1012/L Red blood cells (RBCs) Females: 3.90-5.03 2 1012/L Males: 38.8%-50.0% Hematocrit Females: 34.9%-44.5% Males: 13.5-17.5 g/dL Hemoglobin (Hgb) Females: 12.0-15.5 g/dL Platelets 150-450 2 109/L CHEMISTRY PROFILE3–8 Pages 6-8 Blood urea nitrogen (BUN), serum 7-20 mg/dL Males: 0.74-1.35 mg/dL Creatinine, serum Females: 0.59-1.04 mg/dL Calcium, total, serum 8.8-10.2 mg/dL Glucose, serum (fasting) ≤126 mg/dL Protein, total, serum 6.3-7.9 g/dL Beta2-microglobulin (B2M), serum 1.21-2.70 mcg/mL SERUM PROTEIN ELECTROPHORESIS (SPEP)9 Pages 8-9 Any M spike presence M spike (myeloma gamma globulin) may be abnormal Unit measures: dL=A deciliter is equal to one tenth of a liter; g=A gram is a unit of measurement of mass, or the total amount of matter in an object; L=A liter is a unit of measurement of volume.