This Item Is the Archived Peer-Reviewed Author-Version Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PHOTOGRAMMETRY for FOREST INVENTORY Planning Guidelines
PNC326-1314 Deployment and integration of cost-effective, high spatial resolution, remotely sensed data for the Australian forestry industry PHOTOGRAMMETRY FOR FOREST INVENTORY Planning Guidelines Osborn J.1, Dell M.1, Stone C.2, Iqbal I.1, Lacey M.1, Lucieer A.1, McCoull C.1 1 Discipline of Geography and Spatial Sciences, University of Tasmania 2 Forest Science, NSW Department of Primary Industries Version 1.1: June 2018 Publication: Photogrammetry for Forest Inventory: Planning Guidelines Project Number: PNC326-1314 This work is supported by funding provided to FWPA by the Australian Government Department of Agriculture, Fisheries and Forestry (DAFF). © 2017 Forest & Wood Products Australia Limited. All rights reserved. Whilst all care has been taken to ensure the accuracy of the information contained in this publication, Forest and Wood Products Australia Limited and all persons associated with them (FWPA) as well as any other contributors make no representations or give any warranty regarding the use, suitability, validity, accuracy, completeness, currency or reliability of the information, including any opinion or advice, contained in this publication. To the maximum extent permitted by law, FWPA disclaims all warranties of any kind, whether express or implied, including but not limited to any warranty that the information is up-to-date, complete, true, legally compliant, accurate, non-misleading or suitable. To the maximum extent permitted by law, FWPA excludes all liability in contract, tort (including negligence), or otherwise for any injury, loss or damage whatsoever (whether direct, indirect, special or consequential) arising out of or in connection with use or reliance on this publication (and any information, opinions or advice therein) and whether caused by any errors, defects, omissions or misrepresentations in this publication. -

Aerial Aftermathsaerial Aftermaths
AERIAL AFTERMATHSAERIAL AFTERMATHS WARTIME FROM ABOVE WARTIME FROM ABOVE CAREN KAPLAN CAREN KAPLAN AERIAL AFTERMATHS next wave: new directions in women’s studies — Caren Kaplan, Inderpal Grewal, Robyn Wiegman, Series Editors AERIAL AFTERMATHS war time from above CAREN KAPLAN Duke University Press Durham and London 2018 © 2018 Duke University Press All rights reserved Printed in the United States of Amer i ca on acid- free paper ∞ Designed by Heather Hensley Typeset in Minion Pro by Westchester Publishing Services Library of Congress Cataloging- in- Publication Data Names: Kaplan, Caren, [date–] author. Title: Aerial aftermaths : war time from above / Caren Kaplan. Description: Durham : Duke University Press, 2017. | Series: Next wave | Includes bibliographical references and index. Identifiers:lccn 2017028530 (print) | lccn 2017041830 (ebook) isbn 9780822372219 (ebook) isbn 9780822370086 (hardcover : alk. paper) isbn 9780822370178 (pbk. : alk. paper) Subjects: lcsh: Photographic surveying— History. | Aerial photography— History. | War photography— History. | Photography, Military. Classification:lcc ta593.2 (ebook) | lcc ta593.2 .k375 2017 (print) | ddc 358.4/54— dc23 lc rec ord available at https:// lccn . loc . gov / 2017028530 Duke University Press gratefully acknowledges the support of the University of California, Davis, which provided funds to support the publication of this book. Cover art: Grant Heilman Photography Frontispiece: Thomas Baldwin, “The Balloon Prospect from above the Clouds,” in Airopaidia. Hand- colored etching. Yale Center for British Art, Paul Mellon Collection. For Sofia — CONTENTS acknowl edgments ix Introduction AERIAL AFTERMATHS 1 1. Surveying War time Aftermaths THE FIRST MILITARY SURVEY OF SCOTLAND 34 2. Balloon Geography THE EMOTION OF MOTION IN AEROSTATIC WARTIME 68 3. La Nature à Coup d’Oeil “SEEING ALL” IN EARLY PA NORAMAS 104 4. -

Digitizing Aerial Photography - Understanding Spatial Resolution
Digitizing Aerial Photography - Understanding Spatial Resolution Trisalyn Nelson Mike Wulder Olaf Niemann M.Sc. Candidate Research Scientist Professor University of Victoria Pacific Forestry Centre University of Victoria (250) 721-7349 (250) 363-6090 (250) 721-7329 email: [email protected] [email protected] [email protected] Abstract Accuracy, flexibility, and cost effectiveness are advantages of using digitized aerial photographs as a data source. However, the relationship between the resolution of digital images, and the aerial photographs from which they were derived, must be addressed. In the following paper we consider issues that impact the spatial resolution of photographs and digitized images, and suggest how users can optimize spatial resolution by selecting an appropriate scanning aperture. Optimal scanning aperture can be chosen by considering the camera system resolution, the original photograph’s scale, and the desired pixel size of the digital image. There are optimal scanning resolutions to use when digitizing aerial photographs. Optimizing spatial resolution will maximize the spatial information obtained from the original photographs without generating unnecessarily large file sizes. Introduction Digitized aerial photography is increasingly the scanning aperture, thereby allowing being used as a data source for studying and important image information to be retained and managing environmental elements such as trees file sizes to be minimized. Choice of scanning (Niemann et al., 1999) and forests (Leckie et al., aperture is related to the resolution of the 1999; Niemann et al., 1999). The popularity of original photograph and/or the desired pixel size digitizing aerial photography is a result of the of the digital image. -

Utilization of Photogrammetry During Establishment of Virtual Rock Collection at Aalto University Jeremiasz Merkel
Utilization of photogrammetry during establishment of virtual rock collection at Aalto University Jeremiasz Merkel School of Engineering Master’s thesis Espoo, 30.09.2019 Supervisor Prof. Jussi Leveinen Advisors M.Sc. Mateusz Janiszewski D.Sc. Lauri Uotinen Aalto University, P.O. BOX 11000, 00076 AALTO www.aalto.fi Abstract of the master’s thesis Author Jeremiasz Merkel Title Utilization of photogrammetry during establishment of virtual rock collection at Aalto University. Master Programme European Mining, Minerals and Environmental Programme. Major European Mining Course Code of major ENG3077 Supervisor Prof. Jussi Leveinen Advisors M.Sc. Mateusz Janiszewski, D.Sc. Lauri Uotinen Date 01.09.2019 Number of pages 56+7 Language English Abstract In recent years, we can observe increasing popularity of the term industry 4.0 which is defined as a new level of organization and control over the entire value chain of the life cycle of products. Experts distinguished nine different technologies, which are essential for the development of industry 4.0. One of them is virtual reality, which is used during processes of data visualisation and digitization. These processes can also include geological collections. Due to limited access to different geological spots, the popularity of destructive techniques during rock testing and high complexity of the process of learning geosciences, geologists are looking for new methods of digitization of different samples of rocks and minerals. The aim of this master thesis was to create a virtual collection of selected rocks and minerals using photogrammetry and virtual reality (VR) technology and develop new tool and interactive learning platform for study mineralogy and petrography. -
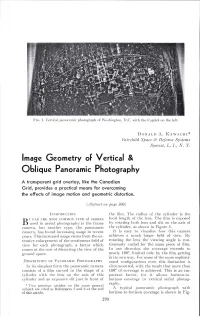
Image Geometry of Vertical & Oblique Panoramic Photography
FIG. 1. Vertical panoramic photograph of Washington, D.C. with the Capitol on the left. DONALD A. KAWACHI* Fairchild Space & Defense Systems Syosset, L. I., N. Y. Image Geometry of Vertical & Oblique Panoramic Photography A transparent grid overlay, like the Canadian Grid, provides a practical means for overcoming the effects of image motion and geometric distortion. (Abstract on page 300) INTRODUCTION the fil m. The radius of the cylinder is the y FAR THE MOST COMMON TYPE of camera focal length of the lens. The fil m is exposed B used in aerial photography is the frame by rotating both lens and slit on the axis of camera, but another type, the panoramic the cylinder, as shown in Figure 3. camera, has found increasing usage in recent I t is easy to visualize how this camera years. This increased usage stems from the ex achieves a much larger field of view. By tensive enlargement of the continuous field of rotating the lens the viewing angle is con view for each photograph, a factor which tinuously varied for the same piece of film. comes at the cost of distorting the view of the In one direction the coverage extends to ground space. nearly 180°, limited only by the film getting in its own way. For some of the more sophisti DESCRIPTION OF PANORAMIC PHOTOGRAPHY cated configurations even this limitation is In its simplest form the panoramic camera circumvented, with the result that more than consists of a film curved in the shape of a 180° of coverage is achieved. This is an im cylinder with the lens on the axis of this portant factor, for it allows horizon-to cylinder and an exposure slit just in front of horizon coverage in vertical aerial photog raphy. -

From God's-Eye to Camera-Eye: Aerial Photography's Post-Humanist and Neo-Humanist Visions of the World
History of Photography ISSN: 0308-7298 (Print) 2150-7295 (Online) Journal homepage: http://www.tandfonline.com/loi/thph20 From God's-eye to Camera-eye: Aerial Photography's Post-humanist and Neo-humanist Visions of the World Paula Amad To cite this article: Paula Amad (2012) From God's-eye to Camera-eye: Aerial Photography's Post-humanist and Neo-humanist Visions of the World, History of Photography, 36:1, 66-86, DOI: 10.1080/03087298.2012.632567 To link to this article: https://doi.org/10.1080/03087298.2012.632567 Published online: 15 Feb 2012. Submit your article to this journal Article views: 1132 View related articles Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=thph20 From God’s-eye to Camera-eye: Aerial Photography’s Post-humanist and Neo-humanist Visions of the World Paula Amad I would like to thank Gordon Beck, Graham Smith, Nick Yablon, and Terry Finnegan for research advice and Lorraine Daston, Kelley Wilder, and Gregg Mitman for their helpful suggestions and for inviting me to present an early version of this essay at the Aerial photographs are most commonly associated with notions of panoptic vision Documenting the World workshop at the or the environmental sublime. This paper reviews the dystopian and utopian dis- Max Planck Institute for the History of courses surrounding aerial photography and suggests a third approach to under- Science, Berlin, in January 2010. standing aerial vision as dialectically situated between the poles of science and art, rationality and imagination, abstracted and embodied knowledge, visibility and Email for correspondence: invisibility, the archive and the museum. -

Cameras and Settings for Aerial Surveys in the Geosciences: Optimizing Image Data
Cameras and settings for aerial surveys in the geosciences: optimizing image data James O’Connor1†, Mike Smith1, Mike R. James2 1 School of Geography and Geology, Kingston University London 2Lancaster Environment Centre, Lancaster University Keywords: UAV, Digital Image, Photography, Remote Sensing Abstract: Aerial image capture has become very common within the geosciences due to the increasing affordability of low payload (<20 kg) Unmanned Aerial Vehicles (UAVs) for consumer markets. Their application to surveying has subsequently led to many studies being undertaken using UAV imagery and derived products as primary data sources. However, image quality and the principles of image capture are seldom given rigorous discussion. In this contribution we firstly revisit the underpinning concepts behind image capture, from which the requirements for acquiring sharp, well exposed and suitable image data are derived. Secondly, the platform, camera, lens and imaging settings relevant to image quality planning are discussed, with worked examples to guide users through the process of considering the factors required for capturing high quality imagery for geoscience investigations. Given a target feature size and ground sample distance based on mission objectives, flight height and velocity should be calculated to ensure motion blur is kept to a minimum. We recommend using a camera with as big a sensor as is permissible for the aerial platform being used (to maximise sensor sensitivity), effective focal lengths of 24 – 35 mm (to minimize errors due to lens distortion) and optimising ISO (to ensure shutter speed is fast enough to minimise motion blur). Finally, we give recommendations for the reporting of results by researchers in order to help improve the confidence in, and reusability of, surveys through: providing open access imagery where possible, presenting example images and excerpts, and detailing appropriate metadata to rigorously describe the image capture process. -

Implementation of Quadcopter for Capturing Panoramic Image at Sedayu Bantul
Proceeding of The 1st International Conference on Computer Science and Engineering 2014|37 Implementation of Quadcopter for Capturing Panoramic Image at Sedayu Bantul Anton Yudhana1, Nuryono Satya Widodo2, Sunardi3 Electrical Engineering Department,Ahmad Dahlan University Jl. Prof. Dr. Soepomo Janturan Umbulharjo Yogyakarta Indonesia [email protected] [email protected] [email protected] Abstract—The aims of this study to deploy an aerial common approach used is similar to overlap and similar light photography system has the ability to capture panoramic image levels to be able to obtain a smooth result. of specific area. The planned research activities completed within 2 years, with the first year's target is Quadcopter design and II. OBJECTIVE manufacture that is equipped with a camera for image acquisition process. Target for the second year is shooting and The main benefit of this research is expected to contribute processing panoramic images thus obtained are accurate. The to the development of science and technology fields of steps to implement this study are divided into two parts: 1) the electrical engineering and in particular for the development of design and manufacture of quadcopter and 2) implementation of sensing systems based quadcopter aerial photographs which image capture and processing. The expected results of the study serve to produce a panoramic image of the rice fields in the is the automated system that have capability of imaging an area area of Sedayu Bantul. and perform image stitching on the imaging results so obtained image represents an area with a particular area with an adequate III. LITERATURE REVIEW level of resolution. -

Applications of Small-Format Aerial Photography in North Dakota James S
Applications of small-format aerial photography in North Dakota James S. Aber Earth Science Department, Emporia State University, Emporia, KS 66801 ([email protected]) Introduction color-visible or color-infrared formats. A variety of camera Modern earth science investigations typically involve a lenses and filters can be applied to achieve special effects. combination of techniques and data types ranging from satellite The camera rigs are operated by radio control from the ground imagery, to ground observations, to subsurface geophysics. to pan (0-360°), tilt (0-90°), and fire the shutter. Single-camera Within this spectrum of observational levels, airphotos are rigs weigh 20 to 40 ounces. commonly used for viewing, mapping, and interpreting natural and cultural resources displayed at the Earth’s surface. For more than half a century, airphotos have been utilized for surveys of various archaeological, biological, and geological resources. For example, airphotos are indispensable aids in the preparation of maps of surficial geology in North Dakota and elsewhere. Conventional aerial photographs normally are acquired from airplanes flying 10,000 to 20,000 feet above the terrain. The photographs are taken with large-format cameras that use film 9 inches (23 cm) wide. Photo scale ranges from 1:10,000 to 1:40,000 and resolution is typically 1-2 meters. Figure 1. Cartoon showing setup for kite aerial photography. The Pictures are taken vertically in overlapping sequence to camera rig is suspended from the kite line and is operated by radio provide complete coverage of large ground areas. The control from the ground. Not to scale. National Aerial Photography Program (NAPP) of the U.S. -

DEVELOPMENT in the FIELD of AERIAL PHOTOGRAPHY , Aelred A
398 PHOTOGRAMMETRIC ENGINEERING will be made, when testing a camera, and the plate will be measured on a two axis comparator. It is believed that not only will accurate calibration of aerial cameras be possible, but it will be fairly convenient to secure distortion data for not only one half a diagonal but for the whole field. This distortion data for the whole field is expected to shed considerable light on the matter of unsymmetrical FIG. 12. Wood scale model of proposed "Hemispherical Collimators."The outside hemisphere will probably be about 9 feet in diameter. The inside hemisphere will probably be about 5 feet in diameter. Approximately 185 two-foot focal length collimators 2 inches in diameter will point upward at a camera mounted at the center of the hemisphere. distortion and the so-called, but little understood, "tangential distortion." This tangential distortion is apparent when it is observed that a line in the image through the principal point is bent. It is also hoped that this device will provide a method of selecting only top quality lenses for mapping cameras. There is still much interesting work to be done; and when this set of colli mators is finished, it is expected that it will be made available to the aerial survey industry for the calibration of cameras. DEVELOPMENT IN THE FIELD OF AERIAL PHOTOGRAPHY , Aelred A. Koepfer Aerial Photography Branch, Photographic Laboratory DEFINITE two-fold developmental objective is being pursued by the A Photographic Laboratory. On one hand, we are progressively improving our present type aerial cameras to fulfill present and near-future requirements. -

Aerial and Close-Range Photogrammetric Technology: Providing Resource Documentation, Interpretation, and Preservation
Suggested citation: Matthews, N. A. 2008. Aerial and Close-Range Photogrammetric Technology: Providing Resource Documentation, Interpretation, and Preservation. Technical Note 428. U.S. Department of the Interior, Bureau of Land Management, National Operations Center, Denver, Colorado. 42 pp. BLM/OC/ST-08/001+9162 Aerial and Close-Range Photogrammetric Technology: Providing Resource Documentation, Interpretation, and Preservation By Neffra A. Matthews Technical Note 428 Bureau of Land Management September 2008 National Operations Center Denver, Colorado 80225 ACKNOWLEDGMENTS I thank my colleagues within the National Operations Center–Division of Resource Services and other offices within BLM who have supported the use of photogrammetry in the Bureau. In addition, I especially thank my coworker, Tom Noble. Several years ago Tom began applying his knowledge of mathematics, surveying, and software programming to photogrammetry. The BLM and the use of close-range photogrammetry in general have greatly benefited from his efforts. I thank the following persons for their reviews of this manuscript: Alan Bell, USDI, Bureau of Reclamation Mike Bies, USDI, Bureau of Land Management Brent Breithaupt, University of Wyoming Matthew Bobo, USDI, Bureau of Land Management Debra Dinville, USDI, Bureau of Land Management Rebecca Doolittle, USDI, Bureau of Land Management Jason Kenworthy, Oregon State University Dave Kett, USDI, Bureau of Land Management Lisa Krosley, USDI, Bureau of Reclamation Lucia Kuizon, USDI, Bureau of Land Management Carolyn McClellan, Smithsonian Institution, National Museum of the American Indian Tom Noble, USDI, Bureau of Land Management Vanessa Stepanak, USDI, Bureau of Land Management Bill Ypsilantis, USDI, Bureau of Land Management CONTENTS Abstract . .1 Introduction..................................................1 What Is Photogrammetry? . -

Introduction to Small-Format Aerial Photography
Chapter 1 Introduction to Small-Format Aerial Photography resources (Warner et al., 1996; Bauer et al., 1997). The Small is beautiful. field is ripe with experimentation and innovation of E. Schumacher 1973, quoted by Mack (2007). equipment and techniques applied to diverse situations. In the past, most aerial photography was conducted from 1.1. OVERVIEW manned platforms, as the presence of a human photogra- pher looking through the camera viewfinder was thought to People have acquired aerial photographs ever since the be essential for acquiring useful imagery. For example, means have existed to lift cameras above the Earth’s Henrard developed an aerial camera in the 1930s, and he surface, beginning in the mid-19th century. Human desire to photographed Paris from small aircrafts for the next four see the Earth ‘‘as the birds do’’ is strong for many practical decades compiling a remarkable aerial survey of the city and aesthetic reasons. From rather limited use in the 19th (Cohen, 2006). century, the scope and technical means of aerial photog- This is still true for many missions and applications raphy expanded throughout the 20th century. The technique today. Perhaps the most famous modern aerial artist- is now utilized for all manners of earth-resource applica- photographer, Y. Arthus-Bertrand, produced his Earth from tions from small and simple to large and sophisticated. above masterpiece by simply flying in a helicopter using Aerial photographs are taken normally from manned hand-held cameras (Arthus-Bertrand, 2002). Likewise, airplanes or helicopters, but many other platforms may be G. Gerster has spent a lifetime acquiring superb photo- used, including balloons, tethered blimps, drones, gliders, graphs of archaeological ruins and natural landscapes rockets, model airplanes, kites, and even birds (Tielkes, throughout the world from the open door of a small airplane 2003).