Spectrum Pharmaceuticals Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

Biocentury 8.15.16
WEEK OF AUGUST 15, 2016 6 PRODUCT DEVELOPMENT: NO ANTIGEN LEFT BEHIND Amgen has added to its immuno-oncology arsenal with its deal for Advaxis’ pan- epitope neoantigen vaccine platform. SERVING RETURNS 9 EMERGING COMPANY PROFILE: RESTRAINING TRANSLATION BY STEVE EDELSON, SENIOR EDITOR Bantam is developing small molecule eIF4E inhibitors to treat a range of cancers, China’s Ally Bridge Group hit the investment world’s starting with B cell malignancies. radar in 2015 with its audacious move to take CRO WuXi PharmaTech Inc. private. If the firm and its partners can engineer the first fruits of that move with REGULATION: the listing of WuXi’s biologics unit in Hong Kong this 10 year, the question is what will be its next moves to ADAPTING FOR THE REAL WORLD generate outsized returns for its investors. EMA says better strategies for real-world evidence and more patient and payer involvement are necessary Ally Bridge, which has a about $1.5 billion under next steps for its adaptive pathway initiative. management across three funds and an international base of LPs, thinks its returns and continued visibility will come from a handful of public and private portfolio companies with transformative data events, the first of EBB & FLOW: 13 which should come this half. LION’S SHARE OF THE WORK Aslan’s validating event. Plus: Cutting a check The firm also says it is working on new private equity to Cleave; and Ironwood’s operating leverage. deals that will rival WuXi in terms of size and impact. Ally Bridge is keeping its private equity plans under wraps, and does not disclose names of investments in its Asia hedge fund. -

2020-2021 Cancer Communications Committee Disclosures All Relationships Are Considered Compensated
2020-2021 Cancer Communications Committee Disclosures All relationships are considered compensated. Relationships are self-held unless otherwise noted. I = Immediate Family Member, Inst = My Institution Name EMAIL Committee Employment Leadership Stock and Other Honoraria Consulting or Advisory Speakers' Bureau Research Funding Patents, Royalties, Other Expert Testimony Travel, Other Relationship (OPTIONAL) (OPTIONAL) Open Member Status Ownership Interests Role Intellectual Property Accommodations, Uncompensated Payments Link Expenses Relationships Neeraj Agarwal [email protected] Active Astellas Pharma Active Biotech (Inst) Astellas Pharma Amgen (Inst) AstraZeneca AstraZeneca (Inst) AstraZeneca Bavarian Nordic (Inst) AVEO Bayer (Inst) Bayer BN ImmunoTherapeutics Bristol-Myers Squibb (Inst) Calithera Biosciences Bristol-Myers Squibb (Inst) Eisai Calithera Biosciences EMD Serono (Inst) Exelixis Celldex (Inst) Foundation Medicine Eisai (Inst) Foundation One Inc Exelixis (Inst) Genentech Genentech (Inst) Janssen Oncology GlaxoSmithKline (Inst) Lilly Immunomedics (Inst) Lilly Janssen (Inst) lily Merck (Inst) Medivation/Astellas Newlink Genetics (Inst) MEI Pharma Novartis (Inst) Merck Pfizer (Inst) Nektar Prometheus (Inst) Novartis Rexahn Pharmaceuticals Pfizer (Inst) Pfizer Sanofi (Inst) Pharmacyclics Takeda (Inst) Seattle Genetics TRACON Pharma (Inst) Muhammad S. Beg muhammad.beg@utsouthwestern. Active Array BioPharma Agios (Inst) edu AstraZeneca/MedImmune ArQule (Inst) Cancer Commons AstraZeneca/MedImmune Ipsen (Inst) Legend Biotech -

Based on Our Discussion with Radford, Management Identified Our Peer Companies to Include the Following 19 Biotechnology and Pharmaceutical Companies for 2015
Based on our discussion with Radford, management identified our peer companies to include the following 19 biotechnology and pharmaceutical companies for 2015: Ariad Pharmaceuticals Inc. Infinity Pharmaceuticals, Inc. Progenics Pharmaceuticals, Inc. Array BiopPharma, Inc. Lexicon Pharmaceuticals, Inc Repligen Corporation Celldex Therapeutics, Inc. MacroGenics, Inc. Spectrum Pharmaceuticals, Inc. CTI BioPharma Corp. Merrimack Pharmaceuticals, Inc. Synta Pharmaceuticals Corp. DepoMed Inc. NewLink Genetics Corporation XOMA Corporation Halozyme Therpeutics, Inc. OncoMed Pharmaceuticals, Inc. Immunomedics Inc. Peregrine Pharmaceuticals, Inc These peer companies were selected from among publicly-held U.S. pharmaceutical and biotechnology companies with comparable operations in mid– to late–stages of product development or small commercial products in the U.S. based on the following criteria: number and stage of development programs; number of employees; market capitalization; and number of and revenue from commercial products. The market data included information as to base salaries, cash bonuses and stock option awards. Use of Compensation Consultants Our Compensation Committee is authorized to retain its own independent advisors to assist in carrying out its responsibilities. Our Compensation Committee engaged Radford to analyze historic compensation and establish recommendations for executive compensation for 2015 and methodologies for determining compensation on an on-going basis. Benchmarking in the Context of Our Other Executive Compensation Principles Our Compensation Committee and our Board of Directors use market data as one means of evaluating and establishing executive pay. In instances where an executive officer is believed to be especially suited to our company or important to our success, the Compensation Committee may establish or recommend compensation that deviates from industry averages or other specific benchmarks. -

JMP Securities Healthcare Conference to Be Held in New York
JMP Securities Healthcare Conference to Be Held in New York SAN FRANCISCO, Oct 01, 2008 (BUSINESS WIRE) -- JMP Group Inc. (NYSE:JMP), an investment banking and alternative asset management firm, announced today that its broker-dealer subsidiary, JMPSecurities, will host the third annual JMP Securities Healthcare Focus Conference at Le Parker Meridien in New York on Monday and Tuesday, October 6 and 7, 2008. The two-day conference will feature presentations by senior executives of nearly 90 public and private companies in the biotechnology, medical devices and healthcare services industries before an audience of institutional investors and financial sponsors. For additional details or to register to attend the event, visit www.jmpsecurities.com/about/events.html or www.twstevent.com/jmp.html. Companies scheduled to present as of September 30, 2008; subject to change: A.D.A.M., Inc. ACADIA Pharmaceuticals Inc. Acceleron Pharma, Inc. Achillion Pharmaceuticals, Inc. Alexza Pharmaceuticals, Inc. Allos Therapeutics, Inc. Allscripts Healthcare Solutions, Inc. Alnylam Pharmaceuticals, Inc. American CareSource Holdings, Inc. Anadys Pharmaceuticals, Inc. ARCA biopharma, Inc. Ardea Biosciences, Inc. athenahealth Inc. AtriCure, Inc. AVEO Pharmaceuticals, Inc. AVI BioPharma, Inc. BioCryst Pharmaceuticals, Inc. C. R. Bard, Inc. Cadence Pharmaceuticals, Inc. Celera Corporation Celgene Corporation Celleration, Inc. Cerus Corporation Chem Rx Corporation Concert Pharmaceuticals, Inc. Corcept Therapeutics Incorporated Cypress Bioscience, Inc. Cytokinetics, Incorporated DexCom, Inc. DiagnoCure Inc. Dialysis Corporation of America Echo Therapeutics, Inc. Eclipsys Corp. Enanta Pharmaceuticals, Inc. Endocare Inc. Exelixis, Inc. EyeGate Pharmaceuticals, Inc. Genomic Health, Inc. Given Imaging, Ltd. HeartWare Limited Horizon Therapeutics, Inc. Human Genome Sciences, Inc. HydroCision, Inc. Idenix Pharmaceuticals, Inc. Idera Pharmaceuticals, Inc. -

Company Description
November 8, 2017 Laura S. Engel, CPA [email protected] 214-987-4121 MARKET STATISTICS COMPANY DESCRIPTION Exchange / Symbol NasdaqGS: SPPI Spectrum Pharmaceuticals, Inc. is focused on the acquisition, development and Price: $19.00 commercialization of proprietary drugs, primarily addressing the oncology/hematology Market Cap ($mm): $1,912.6 markets. Spectrum's business strategy involves in-licensing or acquiring diversified drugs Enterprise Value ($mm): $1,766.6 as well as creating an expanding pipeline of prospective candidates in late-stage Phase 2 and Phase 3 clinical trials. Over the years, the Company has developed comprehensive in- Shares Outstanding (mm): 100.7 house clinical development/regulatory capabilities, along with an extensive commercial Float: 85% network, including a direct sales force in the U.S. and distributors in Europe and Japan Volume (3-month avg., mm): 1.8 for its marketed products. Spectrum’s diverse portfolio consists of six marketed oncology drugs and a pipeline with three advanced stage products that address sizable markets. 52-week Range: $3.73 – $21.95 Spectrum Pharmaceuticals is headquartered in Henderson, Nevada, and as last reported, Industry: Biotechnology the Company had 227 employees. CONDENSED BALANCE SHEET ($mm, except per share data) Balance Sheet Date 9/30/2017 SUMMARY Cash & Cash Equivalents: $247.7 Over the last several quarters, Spectrum’s primary focus has shifted beyond its Cash/Share: $2.46 established portfolio of niche cancer drugs to opportunities for its newer drugs, with three in its late-stage pipeline (Poziotinib, Rolontis™, and Qapzola®), and all of which Debt: $101.8 have meaningful competitive advantages and address indications with significant Equity (Book Value): $304.7 populations. -

13-ICML Abbvie Acerta Pharma Amgen (Europe) Gmbh Bayer
13th INTERNATIONAL CONFERENCE ON MALIGNANT LYMPHOMA Lugano, Switzerland, June 17-20, 2015 13-ICML THE CONFERENCE ORGANIZERS WISH TO THANK THE FOLLOWING SPONSORS FOR THEIR UNRESTRICTED FINANCIAL SUPPORT: Abbvie Acerta Pharma Amgen (Europe) GmbH Bayer HealthCare Pharmaceuticals, Inc. Bristol-Myers Squibb Celgene Corporation CTI Life Sciences Ltd. F. Hoffmann-La Roche Ltd . www.roche.com Gilead Sciences, Inc. Gilead Sciences Europe Ltd. Infinity Pharmaceuticals, Inc. Institut Biochimique SA – IBSA Janssen Pharmaceutical Companies of Johnson & Johnson Medicom Worldwide, Inc. Mentrik Biotech, LLC Mundipharma Oncology www.mundipharma.ch Novartis Oncology Pfizer Oncology Seattle Genetics, Inc. Servier sigma-tau Research Switzerland SA Spectrum Pharmaceuticals, Inc. Takeda Oncology Vifor SA THE CONFERENCE ORGANIZERS WISH TO THANK THE FOLLOWING CONTRIBUTORS FOR THEIR SUPPORT : American Association for Cancer Research – AACR Arcobaleno - Comunità Tariffale Ticino e Moesano CALYM, the Carnot Lymphoma Institute City of Lugano www.lugano.ch/ European School of Oncology – ESO www.eso.net European Society for Medical www.esmo.org Oncology – ESMO European Society for Radiotherapy and Oncology – ESTRO Hotel Splendide Royal www.splendide.ch International Extranodal Lymphoma Study Group - IELSG International Lymphoma Radiation Oncology Group - ILROG Kompetenznetz Maligne Lymphome e.V. – KML www.LHRM.de Leukämiehilfe Rhein-Main g.e.V. - LHRM Lugano MICE Convention Bureau www.luganomice.ch Lugano University Campus http://www.lymphomahub.co Lymphoma HUB m/search/ICML%25202015 Omega SA Repubblica e Cantone Ticino/Fondo Swisslos San Salvatore Foundation Schweizerische Patientenorganisation für Lymphombetroffene und Angehörige - ho / noho Swiss Cancer League Swiss Cancer Research Foundation Swiss International Air Lines The Leukemia Lymphoma Society Ticino Tourism www.ticino.ch . -

IHE Ishares US Pharmaceuticals ETF Gray Swan Event Factor For
ETF Risk Report: IHE Buyer beware: Every ETF holds the full risk of its underlying equities Disclosures in the best interest of investors iShares US Pharmaceuticals ETF Gray Swan Event Risks exist for every equity held by IHE. Gray swan events include accounting fraud, management failures, failed internal controls, M&A problems, restatements, etc. These risks occur infrequently, but Gray Swan Event Factor for IHE 2.72% consistently for all equities. Equities account for 99.86% of IHE’s assets. Most investors ignore these risks until after they are disclosed; whereupon a stock’s price drops precipitously. Just as insurance companies can predict likely costs for a driver’s future car accidents based on the driver’s history, Watchdog Research contacts each ETF asking how they notify investors about we predict the likely cost (price drop) for IHE following accounting governance risks in equities in their fund. We will publish their response gray swan disclosures within its holdings. The expected when received. price decrease across the IHE equity portfolio is 2.72%. However, individual equity risks vary signicantly. This report helps investors know their risk exposure. Inception Date: 05/01/2020 Year-to-Date Return: -0.64% The iShares U.S. Pharmaceuticals ETF tracks the investment results of the Dow Jones U.S. Select Net Assets: $355m 1-Year Return: 33.74% Pharmaceuticals Index, composed of U.S. equities in the pharmaceuticals sector. The Fund uses a passive or Price: $177.65 3-Year Return: 7.84% indexing approach and invests by sampling the Index, Net Asset Value (NAV): $177.38 5-Year Return: 6.78% holding a collection of securities that approximates the full Index in key investment characteristics (such as Net Expense Ratio: 0.42% Yield: 1.23% market capitalization and industry weightings), fundamentals (such as return variability and yield), and As of: 03/31/2021 liquidity. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -
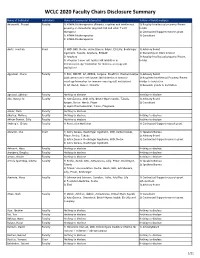
WCLC 2020 Faculty Chairs Disclosure Summary
WCLC 2020 Faculty Chairs Disclosure Summary Name of Individual Individual's Name of Commercial Interest(s) Nature of Relationship(s) Adusumilli, Prasad FacultyRole(s) in Activity 1) ATARA Biotherapeutics (Patents, royalties and intellectual 1) Royalty/Intellectual property/Patent property on mesothelin-targeted CAR and other T-cell holder therapies) 2) Contracted/Support research grant 2) ATARA Biotherapeutics 3) Consultant 3) ATARA Biotherapeutics Aerts, Joachim Chair 1) MSD, BMS, Roche, Astra-Zeneca, BAyer, Eli-Lilly, Boehringer 1) Advisory Board Ingelheim, Takeda, Amphera, BIOCAD 2) Ownership or Stock interest 2) Amphera 3) Royalty/Intellectual property/Patent 3) allogenic tumor cell lysate/JAK inhibition in holder immunooncology/ biomarker for immuno-oncology (all institution) Aggarwal, Charu Faculty 1) BMS, ROCHE, AZ, MERCK, Celgene, BluePrint, Diachaii Sankyo 1) Advisory Board 2)Allogenic tumor cell lysate/JAK inhibition in immuno- 2) Royalties/Intellectual Property/Patent oncology/biomarker for immuno-oncology (all institution) Holder to institution 3) AZ, Merck, Xencor, Novartis 3) Research grants to institution Agrawal, Abhinav Faculty Nothing to disclose Nothing to disclose Ahn, Myung-Ju Faculty 1) AstraZeneca, MSD, Lilly, Bristol-Myers Squibb, Takeda, 1) Advisory Board Amgen, Roche, Merck, Pfizer 2) Consultant 2) Alpha Pharmaceutical, Yuhan, Progenere Aisner, Dara Faculty Nothing to disclose Akerley, Wallace Faculty Nothing to disclose Nothing to disclose Akhtar-Danesh, Gilly Faculty Nothing to disclose Nothing to disclose -

The Bottom 99
The Top 100 January, 2015 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. AWAY Homeaway, Inc. Consumer Discretionary DWA DreamWorks Animation SKG, Inc. Class A Consumer Discretionary DXLG Destination XL Group, Inc. Consumer Discretionary NXST Nexstar Broadcasting Group, Inc. Class A Consumer Discretionary TSLA Tesla Motors, Inc. Consumer Discretionary ZQK Quiksilver, Inc. Consumer Discretionary CMLP Crestwood Midstream Partners LP Energy LNG Cheniere Energy, Inc. Energy SZYM Solazyme, Inc. Energy ACAD ACADIA Pharmaceuticals Inc. Health Care AKRX Akorn, Inc. Health Care ALIM Alimera Sciences, Inc. Health Care ALNY Alnylam Pharmaceuticals, Inc Health Care ARWR Arrowhead Research Corporation Health Care ATHN athenahealth, Inc. Health Care ATHX Athersys, Inc. Health Care AUXL Auxilium Pharmaceuticals, Inc. Health Care BDSI BioDelivery Sciences International, Inc. Health Care CERS Cerus Corporation Health Care CLDX Celldex Therapeutics, Inc. Health Care CPHD Cepheid Health Care CSII Cardiovascular Systems, Inc. Health Care CSU Capital Senior Living Corporation Health Care DRRX DURECT Corporation Health Care DYAX Dyax Corp. Health Care EBIO Eleven Biotherapeutics, Inc. Health Care EGLT Egalet Corporation Health Care EPZM Epizyme, Inc. Health Care EXAS Exact Sciences Corporation Health Care FCSC Fibrocell Science, Inc. Health Care FLDM Fluidigm Corporation Health Care GEVA Synageva BioPharma Corp. Health Care HALO Halozyme Therapeutics, Inc. Health Care ICPT Intercept Pharmaceuticals, Inc. Health Care IDRA Idera Pharmaceuticals, Inc. Health Care INSM Insmed Incorporated Health Care IRWD Ironwood Pharmaceuticals, Inc. Class A Health Care ISR IsoRay, Inc. Health Care KERX Keryx Biopharmaceuticals, Inc. Health Care KPTI Karyopharm Therapeutics, Inc. Health Care KYTH KYTHERA Biopharmaceuticals, Inc. Health Care MDCO Medicines Company Health Care MDSO Medidata Solutions, Inc. -

Oncology Grand Rounds
Oncology Grand Rounds Nurse and Physician Investigators Discuss New Agents, Novel Therapies and Actual Cases from Practice Part 6: Emerging Strategies in Non-Small Cell Lung Cancer CNE Information TARGET AUDIENCE specialists with the formulation of state-of-the-art clinical This activity has been designed to meet the educational management strategies to facilitate optimal care of patients needs of oncology nurses, nurse practitioners and clinical with lung cancer. nurse specialists involved in the treatment of lung cancer. LEARNING OBJECTIVES OVERVIEW OF ACTIVITY • Review the recent FDA approval of anti-PD-L1 antibody Lung cancer is a devastating disease with a broad-reaching consolidation therapy for patients with unresectable Stage impact on public health as it accounts for 14% of all new III NSCLC who have not experienced disease progression cancer cases in the United States and the most cancer-related after standard platinum-based chemotherapy concurrent deaths among both men and women. In the year 2019, it with radiation therapy, and discern how this strategy can is estimated that approximately 228,150 individuals will be be appropriately and safely integrated into routine clinical diagnosed with cancer of the lung and bronchus. Despite the practice. many advances over the past few decades, death rates attrib- • Review recent FDA approvals and other therapeutic utable to lung cancer have remained relatively unchanged. advances related to the long-term management of Today, many are optimistic that these trends have already metastatic NSCLC with an EGFR tumor mutation, and started to change as recent research advances in oncology discern how this information should be applied to current have led to the FDA approval of multiple biologic agents and off-protocol patient care.