Recent Advances in the Detection of Base Modifications Using
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
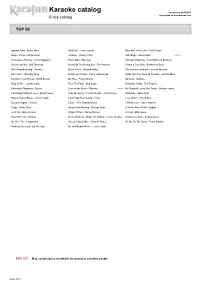
Karaoke Catalog Updated On: 24/04/2017 Sing Online on Entire Catalog
Karaoke catalog Updated on: 24/04/2017 Sing online on www.karafun.com Entire catalog TOP 50 Uptown Funk - Bruno Mars All Of Me - John Legend Blue Ain't Your Color - Keith Urban Shape of You - Ed Sheeran Jackson - Johnny Cash 24K Magic - Bruno Mars EXPLICIT Tennessee Whiskey - Chris Stapleton Piano Man - Billy Joel Unchained Melody - The Righteous Brothers Sweet Caroline - Neil Diamond House Of The Rising Sun - The Animals I Want It That Way - Backstreet Boys Don't Stop Believing - Journey Black Velvet - Alannah Myles Sweet Home Alabama - Lynyrd Skynyrd Girl Crush - Little Big Town Before He Cheats - Carrie Underwood (Sittin' On) The Dock Of The Bay - Otis Redding Friends In Low Places - Garth Brooks My Way - Frank Sinatra Santeria - Sublime Ring Of Fire - Johnny Cash Turn The Page - Bob Seger Killing Me Softly - The Fugees Bohemian Rhapsody - Queen Love on the Brain - Rihanna EXPLICIT He Stopped Loving Her Today - George Jones Can't Help Falling In Love - Elvis Presley Take Me Home, Country Roads - John Denver Wannabe - Spice Girls Folsom Prison Blues - Johnny Cash Can't Stop The Feeling - Trolls Love Shack - The B-52's Summer Nights - Grease Closer - The Chainsmokers I Will Survive - Gloria Gaynor Crazy - Patsy Cline Amarillo By Morning - George Strait A Whole New World - Aladdin Let It Go - Idina Menzel Wagon Wheel - Darius Rucker At Last - Etta James How Far I'll Go - Moana These Boots Are Made For Walkin' - Nancy Sinatra Strawberry Wine - Deana Carter My Girl - The Temptations Sweet Child O'Mine - Guns N' Roses Fly Me To The Moon -

Castilleja Publication of the Wyoming Native Plant Society December 2012, Volume 31(4) Posted at Castilleja Linariifolia
Castilleja Publication of the Wyoming Native Plant Society December 2012, Volume 31(4) Posted at www.wynps.org Castilleja linariifolia In this issue: Desert Yellowhead . 1 Botanists Bookshelf - Intermountain Flora 2A . 3 Growing Native Plants – Short Shrubs . 4 Pocket Guide to Native Plants of Teton County . 6 GLORIA in Wyoming: Beartooth Mtns. and Yellowstone National Park. 6 Halfway Down, Halfway to Go . 8 Desert Yellowhead - One Decade Later By Bonnie Heidel Every plant has a story and some have tomes, if only we could read them start-to-finish! The Desert yellowhead (Yermo xanthocephalus) is the only federally listed plant that is endemic to Wyoming. It was discovered and described by Robert Dorn (Dorn 1991, 2006). Desert yellowhead was listed as Threatened ten years ago (Fish & Wildlife Above: Desert yellowhead (Yermo xanthocephalus) by Jane Service 2002) and critical habitat was designated Dorn. From Dorn (1991). Reprinted with permission from at the one known site not long afterward (Fish & Madroño (California Botanical Society). Wildlife Service 2004). More recently, mining developments in the vicinity have lead to a 20- completed it two years later (Fish & Wildlife year mineral withdrawal and road closures by Service 2010). Subsequently, the Service began the land-managing agency, Bureau of Land to compile information since the time of listing, Management (BLM 2008). called a 5-year review. It was completed and In 2004, a nine-year monitoring saga to posted this fall (Fish & Wildlife Service 2012). annually census the entire population of Desert These reviews revived questions about yellowhead was completed by Richard and species’ habitat requirements and survey Beverly Scott (2009). -

Zombies in Western Culture: a Twenty-First Century Crisis
JOHN VERVAEKE, CHRISTOPHER MASTROPIETRO AND FILIP MISCEVIC Zombies in Western Culture A Twenty-First Century Crisis To access digital resources including: blog posts videos online appendices and to purchase copies of this book in: hardback paperback ebook editions Go to: https://www.openbookpublishers.com/product/602 Open Book Publishers is a non-profit independent initiative. We rely on sales and donations to continue publishing high-quality academic works. Zombies in Western Culture A Twenty-First Century Crisis John Vervaeke, Christopher Mastropietro, and Filip Miscevic https://www.openbookpublishers.com © 2017 John Vervaeke, Christopher Mastropietro and Filip Miscevic. This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: John Vervaeke, Christopher Mastropietro and Filip Miscevic, Zombies in Western Culture: A Twenty-First Century Crisis. Cambridge, UK: Open Book Publishers, 2017, http://dx.doi. org/10.11647/OBP.0113 In order to access detailed and updated information on the license, please visit https:// www.openbookpublishers.com/product/602#copyright Further details about CC BY licenses are available at http://creativecommons.org/licenses/ by/4.0/ All external links were active at the time of publication unless otherwise stated and have been archived via the Internet Archive Wayback Machine at https://archive.org/web Digital material and resources associated with this volume are available at https://www. -

Onderwijsrecensies Basisonderwijs 2015 - 1 4-6 Jaar Prentenboeken
Onderwijsrecensies basisonderwijs 2015 - 1 4-6 jaar Prentenboeken 2014-29-3450 Bon, Annemarie • Hiep hiep Haas! Niveau/leeftijd : AK Winkelprijs : € 14.95 Hiep hiep Haas! / Annemarie Bon ; met illustraties van Gertie Jaquet. - Amsterdam : Bijzonderheden : J1/J2/EX/ Moon, [2014]. - 26 ongenummerde pagina's : illustraties ; 30 cm Volgnummer : 44 / 173 ISBN 978-90-443-4521-6 Nog een nachtje slapen en dan is Haas jarig. Een verjaardagsfeest organiseren kost veel energie en tijd. Als Haas om acht uur naar bed gaat is hij best moe, maar slapen kan hij niet. Dan maar weer zijn bed uit! De hele nacht blijft hij doorgaan, waarbij brokken worden gemaakt en de taart verbrandt. Uitgeput valt hij ten slotte in slaap en wordt pas weer wakker als de feestgangers om zijn bed staan te zingen. Grappig, vlot leesbaar prentenboek met duidelijke tekst. De illustraties, in krijt stift en verf, zijn feestelijk gekleurd en in wisselende afmetingen. Ze zijn speels over de bladzijden verdeeld en sluiten naadloos aan op de situaties. Op elke pagina is een spinnetje verstopt en oplettende kleuters kunnen het tijdsverloop via tal van klokken volgen. Herkenbaar verhaal over de opwinding rondom een verjaardag. Vanaf ca. 4 jaar. Nelleke Hulscher-Meihuizen 2014-27-1933 Heruitgave Burton, Virginia Lee • Het huisje dat verhuisde *zie a.i.'s deze week voor nog vier prentenboeken uit de reeks 'Lemniscaat Het huisje dat verhuisde / Virginia Lee Burton ; vertaling [uit het Engels]: L.M. Niskos. Kroonjuweel'. - Vierde druk. - Rotterdam : Lemniscaat, 2014. - 44 ongenummerde pagina's : illustraties ; 24 × 26 cm. - (Lemniscaat Kroonjuweel). - Vertaling van: The little house. Niveau/leeftijd : AK - Boston : Houghton Mifflin, 1942. -

Gaia, the Planetary Religion : the Sacred Marriage of Art and Science
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-1994 Gaia, the planetary religion : the sacred marriage of art and science. Doctress Neutopia University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Neutopia, Doctress, "Gaia, the planetary religion : the sacred marriage of art and science." (1994). Doctoral Dissertations 1896 - February 2014. 5144. https://scholarworks.umass.edu/dissertations_1/5144 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. GAIA, THE PLANETARY RELIGION: THE SACRED MARRIAGE OF ART AND SCIENCE A Dissertation Presented by DOCTRESS NEUTOPIA Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF EDUCATION February 1994 School of Education GAIA, THE PLANETARY RELIGION: THE SACRED MARRIAGE OF ART AND SCIENCE A Dissertation Presented by DOCTRESS NEUTOPIA Approved as to style and content by: o W. J.Jackson, Dejan 1 of Education Copyright by Doctress Neutopia 1994 All Rights Reserved DEDICATION GAIA The World Soul: Past, Present, and Future ACKNOWLEDGEMENTS My profound thanks to dame Phyllis Rodin for engaging me in long deep conversations about the life-force. I would also like to thank my committee members: Dr. Robert Baker for encouraging me to do a traditional work of scholarship; Dr. Jack Wideman for supporting my efforts after searching for one long year for an outside member; and especially Dr. -

Best of Student Essays
Volunteer State Community College Best of Student Essays from the 2014-2015 Academic Year Expository Essays & Research Writing This publication is made possible with funding from the VSCC Humanities Division, Department of English Faculty, and the support of Bedford/St. Martin’s Publishers. Acknowledgements Dean of Humanities: Alycia Ehlert, Ed.D. English Department Chair: Laura Black Best Essays Committee Chair: Leslie LaChance Best Essays Selection Committee: Laura Black, Jessica Cocita, Mickey Hall, Deborah Moore, Kelly Ormsby, Jaime Sanchez, Cynthia Wyatt Support Staff: Rhonda Custer, Debra Lindsay Editing and Layout: Leslie LaChance Production and Design: Eric Melcher, Coordinator of Communications ii Introduction Volunteer State Community College Best Essays is a new incarnation of a previous publication entitled VSCC English Department’s Best Essays. As the early version has done over the past decade, this publication will continue to showcase some of the best writing being done by students at Volunteer State, and we have just expanded the publication to include examples of excellent writing from a variety of academic disciplines. While much of the work appearing here is nominated by faculty in the Department of English, which publishes this book, faculty from other disciplines are now also invited to nominate student essays for collection. This year, we are pleased to include our first essay from a discipline other than English, “Patriarchy’s Roots,” written by Amy Leu for History 1110, World Civilization 1. Each year, instructors at Volunteer State nominate students who have demonstrated excellence in writing and invite them to submit an essay to our selection committee; that committee of Vol State faculty then works collaboratively to choose superior student work for publication. -

Three Optimized Workflows for Cpg Island Methylation Profiling
APPLICATION NOTE Three Optimized Workflows for CpG Island Methylation Profiling Three Optimized Workflows for CpG Island Methylation Profiling DNA methylation is involved in the Discovery regulation of many cellular processes SOLiD™ Do you know your candidate gene? including X chromosome inactivation, No System chromosome stability, chromatin structure, Yes embryonic development, and transcription. Validation/Screening with Capillary Electrophoresis Do you know your candidate region or In mammals only the 5' carbons of the methylation state of that region? cytosines adjacent to guanines (CpG Yes No dinucleotides: cytosine-phospho-guanine) MSMSA Bisulfite Conversion Bisulfite Conversion are substrates for DNA methylation. (MethylSEQr™ Kit) (MethylSEQr™ Kit) Importance of CpG Islands Are there a high number Bisulfite Specific (BSP) CpG islands are 300−3000 base pair of poly T or A, or large Primer Design fragment size (>300 bps)? (Methyl Primer® Express) stretches of DNA that are CG rich, and are often found within unmethylated Yes No regions of promoters. Profiling of CpG Clone based Sequencing Direct PCR Sequencing island methylation indicates that some Fluorescent BSP PCR genes are more frequently methylated Bisulfite Specific (BSP) Primer Design Bisulfite Specific (BSP) Primer Design Data Analysis (Methyl Primer® Express) (Methyl Primer® Express) than others [1]. Hence, CpG islands play a (GeneMapper® Software v. 4.0) critical role in regulating gene expression. CpG islands found outside promoter BSP PCR BSP PCR Identification of a candidate region or the No regions are commonly methylated, and methylation state of Yes that region? are believed to be responsible for silencing Cloning Yes transcription of repetitive sequences Cycle Sequencing Direct PCR Cycle Sequencing Are there a high number and parasitic sequence elements, such of poly T or A, or large No as viral DNA. -

Helix: Algorithm/Architecture Co-Design for Accelerating Nanopore Genome Base-Calling
Helix: Algorithm/Architecture Co-design for Accelerating Nanopore Genome Base-calling Qian Lou Sarath Janga Lei Jiang [email protected] [email protected] [email protected] Indiana University Bloomington Indiana University - Purdue Indiana University Bloomington University Indianapolis ABSTRACT KEYWORDS Nanopore genome sequencing is the key to enabling personalized nanopore sequencing; base-calling; processing-in-memory medicine, global food security, and virus surveillance. The state-of- ACM Reference Format: the-art base-callers adopt deep neural networks (DNNs) to trans- Qian Lou, Sarath Janga, and Lei Jiang. 2020. Helix: Algorithm/Architecture late electrical signals generated by nanopore sequencers to digital Co-design for Accelerating Nanopore Genome Base-calling. In Proceedings DNA symbols. A DNN-based base-caller consumes 44:5% of to- of the 2020 International Conference on Parallel Architectures and Compilation tal execution time of a nanopore sequencing pipeline. However, Techniques (PACT ’20), October 3–7, 2020, Virtual Event, GA, USA. ACM, New it is difficult to quantize a base-caller and build a power-efficient York, NY, USA, 12 pages. https://doi.org/10.1145/3410463.3414626 processing-in-memory (PIM) to run the quantized base-caller. Al- though conventional network quantization techniques reduce the 1 INTRODUCTION computing overhead of a base-caller by replacing floating-point Genome sequencing [8, 21, 34, 35, 37] is a cornerstone for enabling multiply-accumulations by cheaper fixed-point operations, it sig- personalized medicine, global food security, and virus surveillance. nificantly increases the number of systematic errors that cannot The emerging nanopore genome sequencing technology [15] is be corrected by read votes. The power density of prior nonvolatile revolutionizing the genome research, industry and market due to memory (NVM)-based PIMs has already exceeded memory thermal its ability to generate ultra-long DNA fragments, aka long reads, tolerance even with active heat sinks, because their power efficiency as well as provide portability. -

DNA Methylation Analysis: Choosing the Right Method
biology Review DNA Methylation Analysis: Choosing the Right Method Sergey Kurdyukov 1,* and Martyn Bullock 2 Received: 8 July 2015; Accepted: 22 December 2015; Published: 6 January 2016 Academic Editor: Melanie Ehrlich 1 Genomics Core facility, Kolling Institute of Medical Research, University of Sydney, Sydney 2065, Australia 2 Cancer Genetics Laboratory, Kolling Institute of Medical Research, University of Sydney, Sydney 2065, Australia; [email protected] * Correspondence: [email protected]; Tel.: +61-299-264-756 Abstract: In the burgeoning field of epigenetics, there are several methods available to determine the methylation status of DNA samples. However, choosing the method that is best suited to answering a particular biological question still proves to be a difficult task. This review aims to provide biologists, particularly those new to the field of epigenetics, with a simple algorithm to help guide them in the selection of the most appropriate assay to meet their research needs. First of all, we have separated all methods into two categories: those that are used for: (1) the discovery of unknown epigenetic changes; and (2) the assessment of DNA methylation within particular regulatory regions/genes of interest. The techniques are then scrutinized and ranked according to their robustness, high throughput capabilities and cost. This review includes the majority of methods available to date, but with a particular focus on commercially available kits or other simple and straightforward solutions that have proven to be useful. Keywords: DNA methylation; 5-methylcytosine; CpG islands; epigenetics; next generation sequencing 1. Introduction DNA methylation in vertebrates is characterized by the addition of a methyl or hydroxymethyl group to the C5 position of cytosine, which occurs mainly in the context of CG dinucleotides. -

High-Resolution Bisulfite-Sequencing of Peripheral Blood DNA Methylation in Early-Onset and Familial Risk Breast Cancer Patients
Author Manuscript Published OnlineFirst on June 7, 2019; DOI: 10.1158/1078-0432.CCR-18-2423 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. High-Resolution Bisulfite-Sequencing of Peripheral Blood DNA Methylation in Early- Onset and Familial Risk Breast Cancer Patients Justin Chen1*, Maria K. Haanpää2*, Joshua J. Gruber1,2, Natalie Jäger1, James M. Ford1,2ǂ, and Michael P. Snyder1ǂ 1Department of Genetics 2Department of Medicine, Oncology Division Stanford University, Stanford, CA 94305, USA. * These authors contributed equally to this work. ǂ Co-corresponding authors Running Title: DNA Methylation and High-Risk Breast Cancer Keywords: DNA Methylation; Breast Cancer; Epigenetics; Cancer Predisposition; Allelic Methylation Additional Information: Financial Support: This work used the Genome Sequencing Service Center by Stanford Center for Genomics and Personalized Medicine Sequencing Center, supported by the grant award NIH S10OD020141. MKH is supported by grants from Sigrid Juselius Foundation, Orion Research Foundation, Päivikki ja Sakari Sohlberg Foundation and Instrumentarium Science Foundation. JJG was supported by fellowships from the Jane Coffin Childs Memorial Fund for Medical Research, Stanford Cancer Institute and Susan G. Komen Foundation, as well as funding from ASCO, the Conquer Cancer Foundation and the Breast Cancer Research Foundation. NJ was supported by an EMBO Long-Term Fellowship (ALTF 325-2014). JMF is supported by the BRCA Foundation and the Breast Cancer Research Foundation. MPS is supported by grants from the NIH including a Centers of Excellence in Genomic Science award (5P50HG00773504). Correspondence: Michael P. Snyder James M. Ford Department of Genetics 269 Campus Dr. Stanford University School of Medicine CCSR Room 1115 300 Pasteur Dr. -

HITTING the MARK in CANCER EPIGENETICS Research in Cancer Epigenetics Is Advancing Rapidly
HITTING THE MARK IN CANCER EPIGENETICS Research in cancer epigenetics is advancing rapidly. Here’s an overview of the tools and methods scientists use to drive progress in the field. For by CUSTOM MEDIA ADVERTISEMENT FEATURE ADVERTISEMENT FEATURE says. “We usually put these cytosine ring — is the best look and behave. Illumina’s Andrew Feber, a cancer patients on a watch and understood epigenetic marker methylation array covers geneticist at UCL Cancer HITTING THE MARK wait scheme. We could have today. Methylation typically 850,000 sites on the Institute in London, is one spared her all that therapy.” silences gene transcription genome, and analyses with of those scientists. He aims Pfister’s experience in patterns that are it, he says, “are sufficient to find epigenetic traces IN CANCER EPIGENETICS illustrates the growing characteristic to specific cell to capture the cancer cell’s of bladder cancer in urine, potential of genomic types and tissues. But when basic identity”. an endeavour he says is RESEARCH IN CANCER EPIGENETICS IS ADVANCING RAPIDLY. Here’s an overview of the tools technologies in epigenetics to it silences tumour suppressor Esteller points out that particularly well suited to and methods scientists use to drive progress in the field. contribute to understanding, genes, cancer may appear. methylation arrays have sequencing since it can diagnosing and treating The first epigenetic many positive aspects, such read the often fragmented cancer. Epigenetic technologies — methylation as low-cost and amenability arrangements of DNA modifications to DNA and arrays — emerged about 15 to formalin-fixed, paraffin- letters in liquid specimens. n 2013 Stefan Pfister, RNA regulate how and when years ago with the launch embedded tissue samples. -

Reproductions Supplied by EDRS Are the Best That Can Be Made from the Original Document
DOCUMENT RESUME ED 479 322 PS 031 474 AUTHOR Kerr, Deborah Lynn TITLE "In the Loop" Responses about Looping at the Middle School Level as Seen through Different Lenses. PUB DATE 2002-04-00 NOTE 233p.; Doctor of Education, National-Louis University. PUB TYPE Dissertations/Theses Doctoral Dissertations (041) -- Reports Research (143) EDRS PRICE EDRS Price MF01/PC10 Plus Postage. DESCRIPTORS Case Studies; Developmental Continuity; Educational Practices; Grade 8; *Grouping (Instructional Purposes); *Looping (Teachers); *Middle School Students; *Middle School Teachers; Middle Schools; Parent Attitudes; Student Adjustment; Student Attitudes; Teacher Attitudes; Teacher Student Relationship; Teaching Methods ABSTRACT Looping, or multi-year teaching, is the pedagogical practice of allowing students and teachers to remain together for 2 or more years. This 2-year case study investigated looping practices and perceptions of looping among eighth-gra'de middle school students, teachers, and parents. Data from two middle schools were collected in the second year of looping through interviews with 12 students,4 teachers, and 11 parents; surveys of 214 students,9 teachers, and 75 parents; and videotapes of looping classrooms. Findings revealed that 80 percent of participants reported positive results from looping related to ease of transition into eighth grade, enhanced sense of knowing about the students' needs, better accountability, more sustained peer and teacher-student relationships, more effective curriculum planning, and enhanced parent involvement. Twenty percent of students and parents preferred having different teachers every year. Students believed they should have a choice about looping.(Ten appendices include interview and survey questions and a compilation of student survey responses. Contains 183 references.) (Author/KB) Reproductions supplied by EDRS are the best that can be made from the original document.