Species Limits and Biogeography of Rhynchospiza Sparrows
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comprehensive Multilocus Assessment of Sparrow (Aves: Passerellidae) Relationships ⇑ John Klicka A, , F
Molecular Phylogenetics and Evolution 77 (2014) 177–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication A comprehensive multilocus assessment of sparrow (Aves: Passerellidae) relationships ⇑ John Klicka a, , F. Keith Barker b,c, Kevin J. Burns d, Scott M. Lanyon b, Irby J. Lovette e, Jaime A. Chaves f,g, Robert W. Bryson Jr. a a Department of Biology and Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA 98195-3010, USA b Department of Ecology, Evolution, and Behavior, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA c Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA d Department of Biology, San Diego State University, San Diego, CA 92182, USA e Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA f Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA g Universidad San Francisco de Quito, USFQ, Colegio de Ciencias Biológicas y Ambientales, y Extensión Galápagos, Campus Cumbayá, Casilla Postal 17-1200-841, Quito, Ecuador article info abstract Article history: The New World sparrows (Emberizidae) are among the best known of songbird groups and have long- Received 6 November 2013 been recognized as one of the prominent components of the New World nine-primaried oscine assem- Revised 16 April 2014 blage. Despite receiving much attention from taxonomists over the years, and only recently using molec- Accepted 21 April 2014 ular methods, was a ‘‘core’’ sparrow clade established allowing the reconstruction of a phylogenetic Available online 30 April 2014 hypothesis that includes the full sampling of sparrow species diversity. -

Listas De Aves 2019
List of bird of CUSCO BIRDING - CUSCO 3399 m.a.s.l N° ENGLISH NAME NOMBRE CIENTIFICO 1 Alder Flycatcher Empidonax alnorum 2 American Golden-Plover Pluvialis dominica 3 American Kestrel Falco sparverius 4 Amethyst-throated Sunangel Heliangelus amethysticollis 5 Andean Avocet Recurvirostra andina 6 Andean Cock-of-the-rock Rupicola peruvianus 7 Andean Condor Vultur gryphus 8 Andean Duck Oxyura ferruginea 9 Andean Flicker Colaptes rupicola 10 Andean Goose Oressochen melanopterus 11 Andean Guan Penelope montagnii 12 Andean Gull Chroicocephalus serranus 13 Andean Hillstar Oreotrochilus estella 14 Andean Lapwing Vanellus resplendens 15 Andean Motmot Momotus aequatorialis 16 Andean Negrito Lessonia oreas 17 Andean Parakeet Bolborhynchus orbygnesius 18 Andean Solitaire Myadestes ralloides 19 Andean Swallow Orochelidon andecola 20 Andean Swift Aeronautes andecolus 21 Andean Tinamou Nothoprocta pentlandii 22 Andean Tit-Spinetail Leptasthenura andicola 23 Aplomado Falcon Falco femoralis 24 Ash-breasted Sierra-Finch Phrygilus plebejus 25 Ash-breasted Tit-Tyrant Anairetes alpinus 26 Ashy-headed Tyrannulet Phyllomyias cinereiceps 27 Azara's Spinetail Synallaxis azarae 28 Baird's Sandpiper Calidris bairdii 29 Bananaquit Coereba flaveola 30 Band-tailed Fruiteater Pipreola intermedia 31 Band-tailed Pigeon Patagioenas fasciata 32 Band-tailed Seedeater Catamenia analis 33 Band-tailed Sierra-Finch Phrygilus alaudinus 34 Band-winged Nightjar Systellura longirostris 35 Bank Swallow Riparia riparia 36 Bar-bellied Woodpecker Veniliornis nigriceps 37 Bare-faced -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -
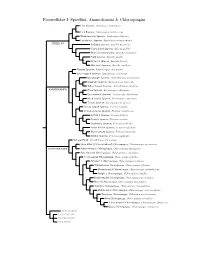
Passerellidae Species Tree
Passerellidae I: Spizellini, Ammodramini & Chlorospingini Lark Sparrow, Chondestes grammacus Lark Bunting, Calamospiza melanocorys Black-throated Sparrow, Amphispiza bilineata Five-striped Sparrow, Amphispiza quinquestriata SPIZELLINI Chipping Sparrow, Spizella passerina Clay-colored Sparrow, Spizella pallida Black-chinned Sparrow, Spizella atrogularis Field Sparrow, Spizella pusilla Brewer’s Sparrow, Spizella breweri Worthen’s Sparrow, Spizella wortheni Tumbes Sparrow, Rhynchospiza stolzmanni Stripe-capped Sparrow, Rhynchospiza strigiceps Grasshopper Sparrow, Ammodramus savannarum Grassland Sparrow, Ammodramus humeralis Yellow-browed Sparrow, Ammodramus aurifrons AMMODRAMINI Olive Sparrow, Arremonops rufivirgatus Green-backed Sparrow, Arremonops chloronotus Black-striped Sparrow, Arremonops conirostris Tocuyo Sparrow, Arremonops tocuyensis Rufous-winged Sparrow, Peucaea carpalis Cinnamon-tailed Sparrow, Peucaea sumichrasti Botteri’s Sparrow, Peucaea botterii Cassin’s Sparrow, Peucaea cassinii Bachman’s Sparrow, Peucaea aestivalis Stripe-headed Sparrow, Peucaea ruficauda Black-chested Sparrow, Peucaea humeralis Bridled Sparrow, Peucaea mystacalis Tanager Finch, Oreothraupis arremonops Short-billed (Yellow-whiskered) Chlorospingus, Chlorospingus parvirostris CHLOROSPINGINI Yellow-throated Chlorospingus, Chlorospingus flavigularis Ashy-throated Chlorospingus, Chlorospingus canigularis Sooty-capped Chlorospingus, Chlorospingus pileatus Wetmore’s Chlorospingus, Chlorospingus wetmorei White-fronted Chlorospingus, Chlorospingus albifrons Brown-headed -

NEWS and NOTES by Paul Hess
NEWS AND NOTES by Paul Hess “Lilian’s” Meadowlark für Ornithologie 135:28), but no details about the findings have been published. Ornithologist Harry C. Oberholser was intrigued by mead - At last, a large-scale genetic report by F. Keith Barker, Ar - owlarks collected in 1929 near Arizona’s Huachuca Moun - ion J. Vandergon, and Scott M. Lanyon arrived in 2008 tains, because their size, structure, and plumage differed (Auk 125:869–879). They examined two mitochondrial from those of the Western Meadowlark and from known DNA (mtDNA) genes and one nuclear locus in 14 Eastern variations of Eastern Meadowlark. In 1930 he described the Meadowlark subspecies throughout the North American, specimens as a new Eastern Meadowlark subspecies and Central American, and South American range. named it lilianae to honor Lilian Baldwin, who had donat - The results indicate a long history of evolutionary diver - ed the collection ( Scientific Publications of the Cleveland Mu - gence between lilianae and all except one other Eastern seum of Natural History 1:83–124). Meadowlark subspecies, auropectoralis of central and We now know it as “Lilian’s” Meadowlark, of desert grasslands in the southwestern U.S. and northern Mexico, where it is distinguishable from Western Meadowlark with careful study by eye and ear. In identification guides, lilianae re - ceived little attention and no illustration until the National Geographic Field Guide to the Birds of North America in 1983. Kevin J. Zimmer of - fered tips in Birding (August 1984, pp. 155–156) and in his books The Western Bird Watcher (Prentice-Hall 1985) and Birding in the American West (Cornell University Press 2000). -

Neotropical Birding 24 2 Neotropical Species ‘Uplisted’ to a Higher Category of Threat in the 2018 IUCN Red List Update
>> FEATURE RED LIST 2018 The 2018 IUCN Red List in the Neotropics James Lowen, Hannah Wheatley, Claudia Hermes, Ian Burfield and David Wege Neotropical Birding 21 featured a summary of the key implications for the Neotropics of the 2016 IUCN Red List for birds. This article briefs readers on the main changes from the 2018 update. s part of its role as the IUCN Red List BirdLife’s Red List team updated the Authority for birds, BirdLife International information available for roughly 2,300 species A is responsible for assessing the global worldwide. Globally, this resulted in changes to conservation status of each of the world’s 11,000 the categorisation of 89 species; 58 species were or so bird species, allocating each to a category ‘uplisted’ to a higher category of threat, whilst ranging from Least Concern to Extinct. The latest roughly half that number – 31 species – were update was published in November 2018 (BirdLife ‘downlisted’. In the Neotropics, 13 species were International 2018). Although much more modest uplisted (Fig. 2) and slightly more – 18 – were in reach than the comprehensive update carried downlisted (Fig. 5). Now let’s take a closer look at out in 2016, whose Neotropical dimension was the individual changes, largely using information discussed in Symes et al. (2017), the 2018 revamp made available on BirdLife’s ‘Globally Threatened contains a suite of interesting changes for species Bird Forums’ (8 globally-threatened-bird- occurring in the Neotropical Bird Club region that forums.birdlife.org). Is the picture quite as rosy as are worth drawing to readers’ collective attention. -

Printable PDF Format
Field Guides Tour Report CENTRAL PERUVIAN ENDEMICS: THE HIGH ANDES 2018 May 31, 2018 to Jun 16, 2018 Dan Lane & Dave Stejskal For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. One of the most-wanted birds of the tour was the Golden-backed Mountain-Tanager. This Peruvian endemic is found in a small area of high forest in the eastern Peruvian Andes, including Bosque Unchog. We had great looks at this beauty at a spot very near our camp. Photo by guide Dave Stejskal. Field Guides has been guiding birding tours to Peru since our inception. Indeed, the first tour ever run at Field Guides back in June of 1985 was to Peru! Since then, we've set up quite a number of diverse offerings to this wonderful, rich country over the years, from the border with Ecuador in the north, south to the Bolivian border, and east into the wilds of western Amazonia. Our most popular offerings seem to be in the northern Andes and the southern Andes (think Marvelous Spatuletail/Long-whiskered Owlet and Machu Picchu/Abra Malaga), but the the vast central Andes region has been relatively neglected all of these years. This expansive region in Peru (in Ancash, Huánuco, Pasco, and Junín departments) holds a high percentage of the total number of Peruvian endemics, a number of which can't be seen anywhere else in the world! But you've really got to want to see these birds in the intensely scenic, high Andean settings where they're found, in order to get through the high elevation hiking, camping (only two nights), and cold temperatures that are endemic to this itinerary. -

Program for 70Th Annual Meeting of the COOPER ORNITHOLOGICAL SOCIETY Riverside, California, 25 - 29 April, 2000
Program and Abstracts Cooper Ornithological Society 70th Annual Meeting 25-29 April 2000 Riverside, California Program for 70th Annual Meeting of the COOPER ORNITHOLOGICAL SOCIETY Riverside, California, 25 - 29 April, 2000 Local Hosts University of California, Riverside Campus University of California Natural Reserve System University of California Cooperative Extension Committee on Local Arrangements John Rotenberry, Chair Tom Scott, Co-chair Paul Aigner Cathy Koehler Karen Bagne Bill Kristan Jutta Burger Michael Patten Sharon Coe Kris Preston Jill Deppe Tim Redman Ian Gillespie Bill Webb Sheila Kee Walter Wehtje Scientific Program Committee Tom Scott, Chair John Rotenberry, Co-chair Charles Collins Kimball Garrett Hugh Ellis Mark Reynolds Steve Zack Sponsors US Geological Survey US Fish and Wildlife Service Region 6 UC Riverside College of Natural and Agricultural Sciences San Bernardino Valley Audubon Society UC Riverside Natural Reserve System UC Cooperative Extension Integrated Hardwood Range Management Program Lynx Edicions Oxford University Press University of Alaska Press Thayer Birding Software UNIVERSITY Habitat Characteristics William D . Berry OF ALASKA of Some Passerine Birds 1954-1956 Alaskan Field Sketches PRESS in Western North American Taiga compiled by Elizabeth Berry • Brina Kessel 1989, paper, reg . $19.95 conf. special $12.00 1998, paper, reg . $16.95 conf, special $13 cloth, reg .95 conf. special $25 To contact us .50 . $39 .00 Over one-third of these eloquent and detailed visit our website: This book describes specific habitat relation- sketches capture a range of Alaska birds . h ttp ://WWW. uaf.ed u/ ships of some of the small land birds that live in the subarctic . It contains descriptions and Accompanied by detailed field notes, the images uapress provide an intimate view of their activities and photographs of typical taiga habitats, color plates of the major bird species discussed, and habitats from hatchlings to adults . -

Proposals 2018-C
AOS Classification Committee – North and Middle America Proposal Set 2018-C 1 March 2018 No. Page Title 01 02 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae 02 10 Split Yellow Warbler (Setophaga petechia) into two species 03 25 Revise the classification and linear sequence of the Tyrannoidea (with amendment) 04 39 Split Cory's Shearwater (Calonectris diomedea) into two species 05 42 Split Puffinus boydi from Audubon’s Shearwater P. lherminieri 06 48 (a) Split extralimital Gracula indica from Hill Myna G. religiosa and (b) move G. religiosa from the main list to Appendix 1 07 51 Split Melozone occipitalis from White-eared Ground-Sparrow M. leucotis 08 61 Split White-collared Seedeater (Sporophila torqueola) into two species (with amendment) 09 72 Lump Taiga Bean-Goose Anser fabalis and Tundra Bean-Goose A. serrirostris 10 78 Recognize Mexican Duck Anas diazi as a species 11 87 Transfer Loxigilla portoricensis and L. violacea to Melopyrrha 12 90 Split Gray Nightjar Caprimulgus indicus into three species, recognizing (a) C. jotaka and (b) C. phalaena 13 93 Split Barn Owl (Tyto alba) into three species 14 99 Split LeConte’s Thrasher (Toxostoma lecontei) into two species 15 105 Revise generic assignments of New World “grassland” sparrows 1 2018-C-1 N&MA Classification Committee pp. 87-105 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae Background: Our current linear sequence of the Accipitridae, which places all the kites at the beginning, followed by the harpy and sea eagles, accipiters and harriers, buteonines, and finally the booted eagles, follows the revised Peters classification of the group (Stresemann and Amadon 1979). -

Behavioral, Morphological, and Ecological Trait Evolution in Two Clades of New World Sparrows (Aimophila and Peucaea, Passerellidae)
Behavioral, morphological, and ecological trait evolution in two clades of New World Sparrows (Aimophila and Peucaea, Passerellidae) Carla Cicero1, Nicholas A. Mason1,4, Lauryn Benedict2 and James D. Rising3,† 1 Museum of Vertebrate Zoology, University of California, Berkeley, Berkeley, CA, United States of America 2 School of Biological Sciences, University of Northern Colorado, Greeley, CO, United States of America 3 Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, Ontario, Canada 4 Current affiliation: Museum of Natural Science and Department of Biological Sciences, Louisiana State University, Baton Rouge, Louisiana, United States of America † Deceased. ABSTRACT The New World sparrows (Passerellidae) are a large, diverse group of songbirds that vary in morphology, behavior, and ecology. Thus, they are excellent for studying trait evolution in a phylogenetic framework. We examined lability versus conservatism in morphological and behavioral traits in two related clades of sparrows (Aimophila, Peucaea), and assessed whether habitat has played an important role in trait evolution. We first inferred a multi-locus phylogeny which we used to reconstruct ancestral states, and then quantified phylogenetic signal among morphological and behavioral traits in these clades and in New World sparrows more broadly. Behavioral traits have a stronger phylogenetic signal than morphological traits. Specifically, vocal duets and song structure are the most highly conserved traits, and nesting behavior appears to be maintained within clades. Furthermore, we found a strong correlation between open habitat and unpatterned plumage, complex song, and ground nesting. However, even within lineages that share the same habitat type, species vary in nesting, plumage pattern, song complexity, and duetting. Our findings highlight trade-offs between behavior, morphology, and ecology in sparrow diversification. -

Birds from Cáceres, Mato Grosso: the Highest Species Richness Ever Recorded in a Brazilian Non-Forest Region
Revista Brasileira de Ornitologia, 24(2), 137-167 ARTICLE June 2016 Birds from Cáceres, Mato Grosso: the highest species richness ever recorded in a Brazilian non-forest region Leonardo Esteves Lopes1,8, João Batista de Pinho2, Aldo Ortiz2, Mahal Massavi Evangelista3, Luís Fábio Silveira4,6, Fabio Schunck4,5,6 and Pedro Ferreira Develey7 1 Laboratório de Biologia Animal, Instituto de Ciências Biológicas e da Saúde, Universidade Federal de Viçosa, Campus Florestal, Rodovia LMG 818, km 6, s/n, CEP 35690-000, Florestal, MG, Brazil. 2 Núcleo de Estudos Ecológicos do Pantanal, Instituto de Biociências, Universidade Federal de Mato Grosso, Avenida Fernando Corrêa da Costa, s/n, Boa Esperança, CEP 78060-900, Cuiabá, MT, Brazil. 3 Faculdade de Ciências Biólogicas, Universidade de Cuiabá, Rua Manoel José de Arruda, 3100, Jardim Europa, CEP 78065-900, Cuiabá, MT, Brazil. 4 Museu de Zoologia, Universidade de São Paulo, Avenida Nazaré, 481, Ipiranga, CEP 04263-000, São Paulo, SP, Brazil. 5 Comitê Brasileiro de Registros Ornitológicos-CBRO, Brazil. 6 Pós-Graduação, Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, Rua do Matão, Travessa 14, 101, Cidade Universitária, CEP 05508-090, São Paulo, SP, Brazil. 7 Sociedade para a Conservação das Aves do Brasil - SAVE, Rua Fernão Dias, 219 cj. 2, Pinheiros, CEP 05427-010, São Paulo, SP, Brazil. 8 Corresponding author: [email protected] Received on 30 January 2016. Accepted on 04 April 2016. ABSTRACT: Them unicipality of Cáceres. Mato Grosso state, Brazil, lies in a contact zone between three semi-arid to arid ecoregions: the Chiquitano Dry Forests, the Cerrado and the Pantanal. -

Species Relationships in the Avian Genus Aimophila
SPECIES RELATIONSHIPS IN THE AVIAN GENUS dIAdOPI-llLd BY LARRY L. WOLF Museumof VertebrateZoology Universityof California ORNITHOLOGICAL MONOGRAPHS NO. 23 PUBLISHED BY THE AMERICAN ORNITHOLOGISTS' UNION 1977 SPECIES RELATIONSHIPS IN THE AVIAN GENUS •IZA4tOPZ--ZZL•I ORNITHOLOGICAL MONOGRAPHS This series,published by the American Ornithologists'Union, has been establishedfor major papers too long for inclusionin the Union's journal, The Auk. Publicationhas been made possiblethrough the generosityof Mrs. Carll Tucker and the Marcia Brady Tucker Foundation,Inc. Correspondenceconcerning manuscripts for publicationin the seriesshould be addressedto the Editor, Dr. John William Hardy, Departmentof Natural Science,The Florida StateMuseum, University of Florida, Gainesville,Florida 32611. Copiesof OrnithologicalMonographs may be orderedfrom the Assistant to the Treasurerof the AOU, Glen E. Woolfender•Department of Biology, Universityof SouthFlorida, Tampa, Florida 33620. (See price list on back and inside back cover.) OrnithologicalMonographs No. 23, viii + 220 pp. Editor of A.O.U. Monographs,John William Hardy SpecialAssociate Editors of this issue,John P. Hubbard, Dela- ware Museum of Natural History, Greenville,Delaware 19807, and Ralph J. Raitt, Departmentof Biology,New Mexico State University,Las Cruces,New Mexico 88001 AssistantEditor, June B. Gabaldon Author, Larry L. Wolf, Departmentof Biology, SyracuseUni- versity, Syracuse,New York 13210 First received, 24 January 1974; accepted,2 February 1976; final revisioncompleted, 9 January 1976 Issued February 23, 1977 Price (includeslong-play phono-discalbum) $12.00 prepaid ($10.50 to AOU Members) Library of CongressCatalogue Card Number 77-73658 Primedby the Allen Press,Inc., Lawrence,Kansas 66044 Copyright ¸ by American Ornithologists'Union, 1977 ii SPECIES RELATIONSHIPS IN THE AVIAN GENUS •1IMOPHIL•I BY LARRY L.