Effects of a Single Bout of Walking on Postprandial Triglycerides in Men of Chinese, European and Japanese Descent: a Multisite Randomised Crossover Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

User Manual (PDF)
D Bedienungsanleitung und Garantieerklärung 1 GB Instruction manual and guarantee 10 F Mode d’emploi et garantie 20 I Manuale di istruzioni e garanzia 30 seca 808 E Manual de instrucciones y garantia 40 DK Betjeningsvejledning og garantibevis 50 S Bruksanvisning och garanti 60 N Bruksanvisning og garantierklæring 70 FIN Käyttöohje ja takuu 80 NL Bedieningshandleiding en garantieverklaring 90 P Instruções de utilização e declaração de garantia 100 GR Οδηγίες χειρισμού και εγγύηση 110 PL Instrukcja obsługi i deklaracja gwarancji 120 RUS seca gmbh & co. kg. 130 Medizinische Waagen und Messsysteme TR Hammer Steindamm 9-25 Kullanım kılavuzu ve garanti beyanı 140 22089 Hamburg J Germany 150 Telephone +49 40 20 00 00 0 Telefax +49 40 20 00 00 50 E-Mail [email protected] seca branches around the world: seca austria seca france seca mexico seca nihon seca north america east seca north america west seca schweiz seca united kingdom seca zhong guo All contact data under www.seca.com Precision for health 1. 2. 3. 4. 5. 6. 7. 1.English Congratulations! By purchasing the seca 808 electronic (proportion of body tissue made up of fat personal scale, you have acquired a high- and water). For this purpose, an imper- ly-accurate and sturdy piece of equip- ceptible, harmless AC current is passed ment. through the body. seca has been putting its experience at The weight display can be switched be- the service of health for over 150 years tween kilogrammes (kg), pounds (lb) and now, and as market leader in many coun- stones (st). Weight is determined within a tries of the world, is always setting new few seconds. -

Medical Measuring Systems and Scales Since 1840
202384016017 medical line 2017 Medical Measuring Systems and Scales Catalog since 1840 North America West seca operates worldwide with headquarters in Germany and branches in: seca corp. 13601 Benson Avenue seca france USA seca united kingdom ٠ CA 91710 ٠ Chino phone +1 800 542 7322 seca north america fax +1 888 705 7397 seca schweiz [email protected] seca zhong guo seca nihon North America East seca mexico seca corp. seca austria 7240 Parkway Drive, Suite 120 seca polska USA ٠ MD 21076 ٠ Hanover seca middle east phone +1 800 542 7322 seca brasil fax +1 888 705 7397 seca suomi [email protected] seca américa latina Germany seca asia pacific and with exclusive partners in seca gmbh & co. kg more than 110 countries. Hammer Steindamm 3 ̶ 25 Germany ٠ Hamburg 22089 phone +49 40 20 00 00 0 All contact data under www.seca.com fax +49 40 20 00 00 50 Every question helps us improve. For more information on seca products please call: 1-800-542-7322 An innovation is something that proves Of course, you can its worth in day-to-day work. also find answers to your questions online at: Doctors, nurses and other occasional use – we offer the www.seca.com health care professionals value right products for a wide range technology that supports them of situations and requirements. in their day-to-day work and The key factor, however, is helps them save time. Our that every seca product forms products help to shorten rou- part of a system solution. -

Medical Measuring Systems and Scales Since 1840
202200002018 medical line + medical line Medical Measuring CATALOG 2018 CATALOG Systems and Scales since 1840 seca gmbh & co. kg Hammer Steindamm 3 ̶ 25 Germany ٠ Hamburg 22089 Telephone +49 40 20 00 00 0 Fax +49 40 20 00 00 50 [email protected] seca operates worldwide with headquarters in Germany and branches in: seca france seca united kingdom seca north america seca schweiz seca zhong guo seca nihon seca mexico seca austria seca polska seca middle east seca brasil seca suomi seca américa latina seca asia pacific and with exclusive partners in more than 110 countries. All contact data at www.seca.com Just ask us. We will find an ‣ Since 1840 we have been the experts in medical answer to your question. measuring and weighing, so we know the challenges Please call us at confronting medical facilities today. On the one hand, +49 40 20 00 00 0 you have to react as a business in which profitability is top priority. Efficient processes and cost awareness are or visit us at critical to maintaining a stable position in the market. www.seca.com On the other hand, you want to ensure the well-being of your patient by meeting high standards for quality, service and innovation, criteria by which hospitals and medical practices are judged in times of the informed patient and an increasingly digitalized world. At seca we demand more of ourselves than a merely adequate reaction to this transformation; we want to have a hand in its development. Our portfolio has long since advanced beyond medical measuring systems and scales and now offers integrated solutions such as measuring stations that communicate with each other, service and software systems that optimize everyday medical processes, and the medical Body Composition Analyzer (mBCA), which uses Bioelectrical Impedance Analysis to revolutionize diagnostics and treatment. -

Seca 856 17-10-07-323C
seca Umschlag 856 A4 quer Seite 1 Dienstag, 3. März 2009 11:04 11 seca 856 seca gmbh & co. kg. Medizinische Waagen und Messsysteme Hammer Steindamm 9-25 22089 Hamburg Germany Telephone +49 40 20 00 00 0 Telefax +49 40 20 00 00 50 E-Mail [email protected] seca branches around the world: seca austria seca france seca mexico seca nihon seca north america east seca north america west seca schweiz seca united kingdom seca zhong guo All contact data under www.seca.com Precision for health D Bedienungsanleitung und Garantieerklärung 4 GB Instruction manual and guarantee 12 F Mode d’emploi et garantie 20 I Manuale di istruzioni e garanzia 28 E Manual de instrucciones y garantia 36 P Instruções de utilização e declaração de garantia 44 1.Deutsch Herzlichen Glückwunsch! Mit der Organ- und Gewebewaage werden. Das Gewicht wird innerhalb weni- seca 856 haben Sie ein hochpräzises und ger Sekunden ermittelt. gleichzeitig robustes Gerät erworben. Mit der Pre-Tara-Funktion können Sie das Seit über 150 Jahren stellt seca seine Er- Gewicht einer Schale speichern. Dieses fahrung in den Dienst der Gesundheit und Gewicht wird bei der Wägung automa- setzt als Marktführer in vielen Ländern der tisch abgezogen. Welt mit innovativen Entwicklungen für Zusätzlich verfügt die Waage über eine das Wiegen und Messen immer neue HOLD-Funktion, um den ermittelten Ge- Maßstäbe. wichtswert zu speichern und über eine Die Organ- und Gewebewaage seca 856 TARA-Funktion, damit Zusatzgewichte kommt entsprechend den nationalen Vor- (z.B. eine Schale) beim Wiegen nicht be- schriften hauptsächlich in Krankenhäusern, rücksichtigt werden. -
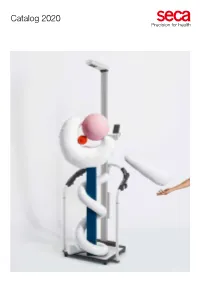
SECA Scale-Up Line (EMR Validate Handrail Scale with ID
Catalog 2020 The future in focus since 1840 The simple, yet vital, daily routine of patient measuring and weighing is performed in every clinical setting, with weight and height measurements remaining as essential cornerstones of clinical care. Our corporate vision is to provide even more necessary vital parameters from a single source – with solutions as trustworthy as our tradition in medical measuring systems and scales. + World market leader in medical measuring and weighing + Made in Germany since 1840 This catalog reinforces our vision in providing a comprehensive product portfolio. In addition to our + Family-owned and operated in the fourth generation established scale selection, you will find bioimpedance solutions that have been rigorously tested by + Exclusive partner and dealer network in over 110 countries medical validation studies, unmatched, certified EMR integration solutions and enhanced vital signs + User-oriented and groundbreaking solutions from our own development department monitors to streamline your workflow. At the same time, seca is committed to continuous software + EMR integration – validated and certified seca products equipped for the future development to bring the perfect product today, tomorrow and in the future. Let yourself be inspired by our innovations and discover the best-suited solution +49 40 20 00 00 0 +971 564 410 292 +60 173 711 973 for your clinical needs today. seca headquarters seca middle east seca asia pacific Robert M. Vogel Frederik Vogel Thomas Wessels CEO CEO CEO Sales & Development & Finance & Marketing Manufacturing Services seca.com Handrail scales Firm support for safe weighing. Handrail scales from seca offer every patient a secure grip and are the best fall prevention when weighing. -

Catalog 2020 the Future in Focus Since 1840
Catalog 2020 The future in focus since 1840 The simple, yet vital, daily routine of patient measuring and weighing is performed in every clinical setting, with weight and height measurements remaining as essential cornerstones of clinical care. Our corporate vision is to provide even more necessary vital parameters from a single source – with solutions as trustworthy as our tradition in medical measuring systems and scales. + World market leader in medical measuring and weighing + Made in Germany since 1840 This catalog reinforces our vision in providing a comprehensive product portfolio. In addition to our + Family-owned and operated in the fourth generation established scale selection, you will find bioimpedance solutions that have been rigorously tested by + Exclusive partner and dealer network in over 110 countries medical validation studies, unmatched, certified EMR integration solutions and enhanced vital signs + User-oriented and groundbreaking solutions from our own development department monitors to streamline your workflow. At the same time, seca is committed to continuous software + EMR integration – validated and certified seca products equipped for the future development to bring the perfect product today, tomorrow and in the future. Let yourself be inspired by our innovations and discover the best-suited solution +49 40 20 00 00 0 +971 564 410 292 +60 173 711 973 for your clinical needs today. seca headquarters seca middle east seca asia pacific Robert M. Vogel Frederik Vogel Thomas Wessels CEO CEO CEO Sales & Development -

2016 Goods Awarded Contracts 2016
2016 GOODS AWARDED CONTRACTS 2016 Contract Contract Purchase Order Date of Contract Reference WHO Office: Supplier Name: Supplier Country: Amount for the supply of: Signature: Number: USD: 201522758 Vaccines Headquarters UNICEF Denmark 16-Jun-2016 $2,664,116.00 201467502 Vaccines Headquarters UNICEF Denmark 01-Apr-2016 $2,612,627.00 201535064 Equipment, Auxiliary, Hospital Eastern Mediterranean Office GAMBRO LUNDIA AB France 11-Jul-2016 $2,179,148.23 201639129 Emergency Health Kits Eastern Mediterranean Office MEDICAL EXPORT GROUP Netherlands 01-Dec-2016 $2,024,231.73 201428856 Vehicles Africa Office TOYOTA MOTOR CORPORATION Japan 24-May-2016 $1,847,472.66 201414166 Emergency Health Kits Eastern Mediterranean Office MEDICAL EXPORT GROUP Netherlands 26-Jan-2016 $1,772,125.00 201530184 Vehicles Eastern Mediterranean Office ALMOTAKAMLEH FOR SPECIALIZED ASSEMBLY & SUPPLY Jordan 28-Jun-2016 $1,650,000.00 201529437 Vaccines Headquarters UNICEF Denmark 27-Jun-2016 $1,493,407.39 201387154 Emergency Health Kits Africa Office MEDICAL EXPORT GROUP Netherlands 15-Apr-2016 $1,325,000.00 201471796 Haemodialysis and Accessories Eastern Mediterranean Office KADECO FZE United Arab Emirates 31-May-2016 $1,321,064.55 201454048 Vehicles Headquarters THE ARMORED GROUP, LLC United States of America 14-Mar-2016 $1,152,669.20 201528458 Emergency Health Kits Eastern Mediterranean Office MEDICAL EXPORT GROUP Netherlands 05-Sep-2016 $1,105,768.60 FIOTEC – FUNDAÇÃO PARA O DESENVOLVIMENTO CIENTÍFICO E 201390282 Vaccines Headquarters Brazil 19-Feb-2016 $1,051,754.39 TECNOLÓGICO EM SAÚDE 201465165 Information Technology Equipment Africa Office DANOFFICE Denmark 28-Apr-2016 $939,130.65 201513685 Vaccines Eastern Mediterranean Office SANOFI PASTEUR INTERNATIONAL France 22-Jun-2016 $850,340.14 United Kingdom of 201653341 Miscellaneous Drugs Eastern Mediterranean Office DURBIN PLC Great Britain and 29-Dec-2016 $785,500.00 Northern Ireland 201486680 Cold Chain, Refrigeration, Air Conditioning Africa Office B MEDICAL SYSTEMS S.À R.L. -

Risk Factors for Breast Cancer in Gaza Strip, Palestine: a Case-Control Study
Clin Nutr Res. 2017 Jul;6(3):161-171 https://doi.org/10.7762/cnr.2017.6.3.161 pISSN 2287-3732·eISSN 2287-3740 CLINICAL NUTRITION RESEARCH Original Article Risk Factors for Breast Cancer in Gaza Strip, Palestine: a Case-Control Study Mueen Kariri ,1 Marwan O. Jalambo ,2 Basil Kanou ,1 Saleh Deqes ,1 Samaher Younis ,1 Baker Zabut ,3 Usama Balawi 1 1Ministry of Health, Gaza, Palestine 2Faculty of Pharmacy, Al-Azhar University-Gaza, Gaza, Palestine 3Biochemistry Department, Islamic University of Gaza, Gaza, Palestine Received: May 24, 2017 ABSTRACT Revised: Jun 26, 2017 Accepted: Jul 10, 2017 Breast cancer (BC) is the main common cause of cancer mortality among women in the Correspondence to world. This study aims at investigating BC epidemiology and identifying the different risk Marwan O. Jalambo factors associated and the most affecting ones among women in the Gaza Strip, Palestine. Faculty of Pharmacy, Al-Azhar University-Gaza, This study was a hospital-based case-control (1:2), as the study was carried out over the Jamal Abdl Naser St., P.O. Box 1277, Gaza, Gaza period of October 2014 to February 2015. A total of 105 BC patients, chosen from Al-Shifa Strip, Palestine. Hospital in Gaza City and European hospital for the south governorate, were the case and Tel: +970-08-2641884 Fax: +970-08-2641888 compared to 209 women as a control group who matched the cases in age, residence, and E-mail: [email protected] with no history of breast problems. The age of the enrolled cases and controlled ranged between 18 to 60 years. -
Medical Measuring Systems and Scales Since 1840
202384016014 medical line Medical Measuring Systems and Scales Catalog 2014 since 1840 North America West seca operates worldwide with headquarters in Germany and branches in: seca corp. 13601 Benson Avenue seca france USA seca united kingdom ٠ CA 91710 ٠ Chino phone +1 800 542 7322 seca north america fax +1 888 705 7397 seca schweiz [email protected] seca zhong guo seca nihon North America East seca mexico seca corp. seca austria 7240 Parkway Drive, Suite 120 seca polska USA ٠ MD 21076 ٠ Hanover seca middle east phone +1 800 542 7322 seca brasil fax +1 888 705 7397 seca suomi [email protected] and with exclusive partners in more than 110 countries. Germany seca gmbh & co. kg Hammer Steindamm 9 ̶ 25 All contact data under www.seca.com Germany ٠ Hamburg 22089 phone +49 40 20 00 00 0 fax +49 40 20 00 00 50 Every question helps us improve. For more information on products and seca Service, please call: 1-800-542-7322 An innovation is something that proves Of course, you can its worth in day-to-day work. also find answers to your questions online at: Doctors, nurses and other occasional use – we offer the www.seca.com health care professionals value right products for a wide range technology that supports them of situations and requirements. in their day-to-day work and The key factor, however, is helps them save time. Our that every seca product forms products help to shorten rou- part of a system solution. This tine procedures and prevent is the only way to ensure that errors. -
Download File
FEATURE Supply Annual Supply financing solutions for children Interventions to meet increased demand for affordable life- saving supplies in a rapidly Report 2015 changing global context Cover photo Nepal: A porter carries UNICEF-provided vaccines to a health post in Gorkha District – the epicentre of the earthquake on 25 April 2015 – for a measles, rubella and polio vaccination campaign Vanuatu: Students sit inside a UNICEF tent being used as a temporary classroom after their school was badly damaged by Cyclone Pam on 13 March 2015 Bolivia: Maribel, 8, and Shirley, 6, wash their hands at a hand- washing station in the Guaraní community. The girls wear the traditional dress of the indigenous Guaraní About UNICEF UNICEF promotes the rights and wellbeing of every child in everything we do. Together with our partners, we work in 190 countries and territories to translate that commitment into practical action, focusing special effort on reaching the most vulnerable and excluded children, to the benefit of all children, everywhere. FOR MORE INFORMATION ABOUT UNICEF AND ITS WORK, PLEASE VISIT WWW.UNICEF.ORG UNICEF Supply Annual Report 2015 Contents 3 Contents Influencing markets Strategic procurement and market and price transparency improved supply security and affordability of key products for children Page 14 FEATURE Financing in-depth Supply financing Savings 2015 Pre-financing Page 9 solutions for children Savings of $422.8 million achieved in 2015 Increasing governments’ Page 11 took total savings to $1.068 billion since 2012 An expanding -

Conference Program
VIRTUAL IGD 2021 SCIENTIFIC PROGRAM IGDC Scientific Workshop Program Timings Tuesday 16th of March 2021 Start End Zoom Online Platform 14:30 15:00 Opening Remarks (Chair of the Conference) H.E. Sheikh Nahayan Mabarak Al Nahayan Cabinet Member, Minister of Tolerance and Coexistence 15:00 15:30 Plenary Session: Omega-3 Fatty Acids for Optimal Health Across the Life Course Prof. Philip Calder Professor of Nutritional Immunology, Faculty of Medicine, University of Southampton, Southampton, United Kingdom Session 1 Ms. Wafaa Helmi Ayesh 15:30 15:50 Oral Tolerance: Myth or Reality! Prof. Joseph Haddad Professor of Pediatrics St. George University Hospital & University of Balamand, Lebanon 15:50 16:10 Role of Public-Private Partnerships in Addressing Childhood Obesity: Evidence from the Implementation and Regional Replication of Ajyal Salima Dr. Carla Habib Lecturer, Faculty of Agriculture & Food Sciences American University Beirut, Lebanon 16:10 16:30 Why Colostrum Prof. Wael Bahbah Professor and Consultant of Pediatrics, Pediatric Clinical Nutrition, Faculty of Medicine, Menoufia University, Egypt 16:30 16:50 Failure to Thrive: The Clinical Tips! Dr. Eslam El-Baroudy Professor & Consultant Pediatrics, Faculty of Medicine, Cairo University, Consultant Pediatrician, Sheikh Khalifa Medical City (SKMC), United Arab Emirates 16:50 18:00 Exhibition Hall / Lounge / Break Oral Abstract Presentation (Exhibition Hall) Sponsored Session 17:15 17:30 TUAT Session Dr. Hiroki Wakamatsu CEO, Tokyo University of Agriculture and Technology Research Institute Co. Ltd, Japan IGDC Scientific Workshop Program Timings Tuesday 16th of March 2021 Start End Zoom Online Platform Session 2 Moderator - Dr. Amita Attlee 18:00 18:20 Metabolic Syndrome in Children and their Relationship to Fat Belly Diseases Dr. -

Seca 354/364 A3
Technische Daten Caractéristiques techniques Datos técnicos seca 354 „lena“ seca 354 « lena » seca 354 « lena » Höchstlast: 20 kg / 44 lbs Charge maximale : 20 kg / 44 lbs Carga máxima: 20 kg / 44 lbs Feineinteilung: 0,01 kg < 10 kg > 0,02 kg Graduation : 0,01 kg < 10 kg > 0,02 kg Divisiones: 0,01 kg < 10 kg > 0,02 kg 0.5 oz < 22 lbs> 1oz 0.5 oz < 22 lbs> 1oz 0.5 oz < 22 lbs> 1oz 0.02 lbs < 22 lbs > 0.05 lbs 0.02 lbs < 22 lbs > 0.05 lbs 0.02 lbs < 22 lbs > 0.05 lbs Genauigkeit: ±20 g oder ±0,4% Précision : ±20 g ou ±0,4% Precisión: ±20 g / ±0,4% Zifferngröße: 22 mm Zifferngröße: 22 mm Tamaño de cifras: 22 mm Abmessungen (B x H x T): 550 x 165 x 330 mm Dimensions (L x H x P) : 550 x 165 x 330 mm Medidas (An x Al x F): 550 x 165 x 330 mm Gesamtgewicht: ca. 2,5 kg Poids total : ca. 2,5 kg Tara: approx. 2,5 kg Temperaturbereich: + 10 °C bis + 40 °C Limites de température : + 10 °C à + 40 °C Gama de temperatura: + 10 °C hasta + 40 °C Batterien: 4 x Typ Mignon AA (1,5V) Piles : 4 x Typ Mignon AA (1,5V) Pilas:: 4 x tipo Mignon AA (1,5V) seca 364 „laetitia“ seca 364 « laetitia » seca 364 « laetitia » Höchstlast: 50 kg / 110 lbs Charge maximale : 50 kg / 110 lbs Carga máxima: 50 kg / 110 lbs Feineinteilung: 0,02 kg < 20 kg > 0,05 kg Graduation : 0,02 kg < 20 kg > 0,05 kg Divisiones: 0,02 kg < 20 kg > 0,05 kg 1 oz < 44 lbs > 2oz 1 oz < 44 lbs > 2oz 1 oz < 44 lbs > 2oz 0,05 lbs < 44lbs > 0,1lbs 0,05 lbs < 44lbs > 0,1lbs 0,05 lbs < 44lbs > 0,1lbs Genauigkeit: ±50 g oder ±0,4% Précision : ±50 g ou ±0,4% Precisión: ±50 g / ±0,4% Sonstige Daten wie