Comparing Oxime-Mediated Reactivation of Nerve Agent Inhibited Acetylcholinesterase from Various Species Maggie Weese Mentored by Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download S/2013/735
United Nations A/68/663–S/2013/735 General Assembly Distr.: General 13 December 2013 Security Council Original: English General Assembly Security Council Sixty-eighth session Sixty-eighth year Agenda item 33 Prevention of armed conflict Identical letters dated 13 December 2013 from the Secretary-General addressed to the President of the General Assembly and the President of the Security Council I have the honour to convey herewith the final report of the United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic (see annex). I would be grateful if the present final report, the letter of transmittal and its appendices could be brought to the attention of the Members of the General Assembly and of the Security Council. (Signed) BAN Ki-moon 13-61784 (E) 131213 *1361784* A/68/663 S/2013/735 Annex Letter of transmittal Having completed our investigation into the allegations of the use of chemical weapons in the Syrian Arab Republic reported to you by Member States, and further to the report of the United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic (hereinafter, the “United Nations Mission”) on allegations of the use of the chemical weapons in the Ghouta area of Damascus on 21 August 2013 (A/67/997-S/2013/553), we have the honour to submit the final report of the United Nations Mission. To date, 16 allegations of separate incidents involving the use of chemical weapons have been reported to the Secretary-General by Member States, including, primarily, the Governments of France, Qatar, the Syrian Arab Republic, the United Kingdom of Great Britain and Northern Ireland and the United States of America. -

Nomination Background: 1-Butyl-3-Methylimidazolium Chloride
Ionic Liquids 1-Butyl-3-methylimidazolium Chloride (CAS No. 79917-90-1) 1-Butyl-1-methylpyrrolidinium Chloride (CAS No. 479500-35-1) N-Butylpyridinium Chloride (CAS No. 1124-64-7) Review of Toxicological Literature May 2004 Ionic Liquids 1-Butyl-3-methylimidazolium Chloride (CAS No. 79917-90-1) 1-Butyl-1-methylpyrrolidinium Chloride (CAS No. 479500-35-1) N-Butylpyridinium Chloride (CAS No. 1124-64-7) Review of Toxicological Literature Prepared for National Toxicology Program (NTP) National Institute of Environmental Health Sciences (NIEHS) National Institutes of Health U.S Department of Health and Human Services Contract No. N01-ES-35515 Project Officer: Scott A. Masten, Ph.D. NTP/NIEHS Research Triangle Park, North Carolina Prepared by Integrated Laboratory Systems, Inc. Research Triangle Park, North Carolina May 2004 Toxicological Summary for Ionic Liquids 05/2004 Abstract Ionic liquids are salts of organic cations with melting points generally below 100 °C and are being widely investigated as replacements for volatile organic solvents in industrial and laboratory processes because they are thought to be "environmentally benign." Although some efforts have begun to study their potential for ecotoxicity, limited vertebrate or genetic toxicity testing has been done. Three ionic liquids, 1-butyl-3-methylimidazolium chloride ([bmim]Cl), 1-butyl-1-methylpyrrolidinium chloride ([bmpy]Cl), and N-butylpyridinium chloride ([NBuPy]Cl), were nominated to the National Toxicology Program (NTP) for toxicological testing based on their widespread interest as possible alternatives to organic solvents. These chlorides are representative of the three most common cation classes of ionic liquids being investigated: imidazolium, pyridinium, and pyrrolidinium. The chlorides, soluble in water and polar organic liquids, are generally prepared from approximately equimolar amounts of the appropriately substituted heterocyclic compound and butyl chloride, often under both heat and pressure. -

(12) Patent Application Publication (10) Pub. No.: US 2012/0046244 A1 Rogers Et Al
US 20120046244A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0046244 A1 Rogers et al. (43) Pub. Date: Feb. 23, 2012 (54) DUAL FUNCTIONING IONIC LIQUIDS AND (86). PCT No.: PCT/USO9/69652 SALTS THEREOF S371 (c)(1), (75) Inventors: Robin D. Rogers, Tuscaloosa, AL (2), (4) Date: Nov. 3, 2011 (US); Daniel T. Daly, Tuscaloosa, AL (US); Douglas MacFarlane, Related U.S. Application Data SG (KSR,kiSt. Port (60) Eyal application No. 61/141,168, filed on Dec. Seddon, Donaghadee (IE): s Gabriela Gurau, Tuscaloosa, AL O O (US); Katharina Bica, Vienna Publication Classification (AT); Jelena Turanjanin, Victoria (51) Int. Cl. (AU); Pamela M. Dean, Victoria A6II 3/66 (2006.01) (AU) A6II 3L/205 (2006.01) A6II 3/545 (2006.01) (73) Assignees: THE BOARD OF TRUSTEES OF A613/606 (2006.01) THE UNIVERSITY OF (52) U.S. Cl. ......... 514/75; 514/166; 514/555; 514/226.2 ALABAMA, Tuscaloosa, AL (US); QUEENS UNIVERSITY (57) ABSTRACT BELFAST, Belfast (UK); MONASH UNIVERSITY, Disclosed herein are ionic liquid compositions comprising Melbourne (AU) active pharmaceutical, biological, and nutritional com pounds, and methods of use. Further disclosed are composi (21) Appl. No.: 13/142.559 tions of matter including liquid ion pairs alone or in Solution and their use; compositions of ionic liquids that are solvated. (22) PCT Filed: Dec. 29, 2009 for example, hydrated and their uses. Patent Application Publication Feb. 23, 2012 Sheet 1 of 7 US 2012/0046244 A1 O 20 40 SO 8) O) Wit% Choline DPP Fig. 1 Patent Application Publication Feb. 23, 2012 Sheet 2 of 7 US 2012/0046244 A1 CN- P(Busal H, Vam 4. -
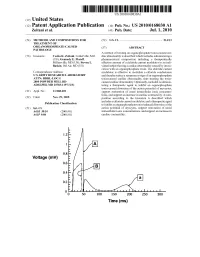
Time (Ms) Patent Application Publication Jul
US 20100168030A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0168030 A1 Zoltani et al. (43) Pub. Date: Jul. 1, 2010 (54) METHODS AND COMPOSITIONS FOR (52) U.S. c1. ........................................................ .. 514/13 TREATMENT OF ORGANOPHOSPHATE-CAUSED (57) ABSTRACT PATHOLOGY A method of treating an organophosphate toxin-caused car (76) Inventors: Csaba K. Zoltani, Lutherville, MD diac abnormality is described Which includes administering a (US); Gennady E. Platoff, pharmaceutical composition including a therapeutically Millersville, MD (US); Steven I. effective amount of a chloride current modulator to an indi Baskin, Bel Air, MD (US) vidual subject having a cardiac abnormality caused by intoxi cation With an organophosphate toxin. The chloride current Correspondence Address: modulator is effective to modulate a chloride conductance U S ARMY RESEARCH LABORATORY and thereby reduce a symptom or sign of an organophosphate ATTN: RDRL-LOC-I toxin-caused cardiac abnormality, thus treating the toxin 2800 POWDER MILL RD caused cardiac abnormality. Optionally, included is adminis ADELPHI, MD 20783-1197 (US) tering a therapeutic agent to inhibit an organophosphate toxin-caused distortion of the action potential of myocytes, (21) App1.No.: 11/288,269 support restoration of usual intracellular ionic concentra tions, and support an increase in cardiac contractility. A com (22) Filed: Nov. 29, 2005 position according to the invention is described Which includes a chloride current modulator; and a therapeutic agent Publication Classi?cation to inhibit an organophosphate toxin-induced distortion of the (51) Int. Cl. action potential of myocytes, support restoration of usual A61K 38/16 (2006.01) intracellular ionic concentrations, and support an increase in A61P 9/06 (2006.01) cardiac contractility. -

Chapter 7 NERVE AGENT BIOSCAVENGER: DEVELOPMENT of a NEW APPROACH to PROTECT AGAINST ORGANO- PHOSPHORUS EXPOSURE † ‡ Michelle C
Nerve Agent Bioscavenger: Development of a New Approach to Protect Against Organophosphorus Exposure Chapter 7 NERVE AGENT BIOSCAVENGER: DEVELOPMENT OF A NEW APPROACH TO PROTECT AGAINST ORGANO- PHOSPHORUS EXPOSURE † ‡ MICHELLE C. ROSS, DVM, PHD*; CLARENCE A. BROOMFIELD, PHD ; DOUGLAS M. CERASOLI, PHD ; § ¥ ¶ ** BHUPENDRA P. DOCTOR, PHD ; DAVID E. LENZ, PHD ; DONALD M. MAXWELL, MS ; AND ASHIMA SAXENA, PHD INTRODUCTION PLASMA-DERIVED STOICHIOMETRIC BIOSCAVENGERS Cholinesterases Pharmacokinetics and the Safety of Plasma-Derived Human Butyrylcholinesterase In Vitro and In Vivo Stability of Plasma-Derived Human Butyrylcholinesterase Efficacy of Plasma-Derived Human Butyrylcholinesterase Immunological Safety of Plasma-Derived Butyrylcholinesterase Behavioral Safety of Plasma-Derived Butyrylcholinesterase RECOMBINANT STOICHIOMETRIC BIOSCAVENGERS CATALYTIC BIOSCAVENGERS INTERAGENCY PARTNERSHIPS: PROJECT BIOSHIELD SUMMARY * Colonel, US Army; Director of CBRN Medical Defense Policy, Office of the Assistant Secretary of Defense for Health Affairs, 5111 Leesburg Pike, Skyline 5, Falls Church, Virginia 22041 † Research Chemist, Research Division, Department of Pharmacology, US Army Medical Research Institute of Chemical Defense, 3100 Ricketts Point Road, Aberdeen Proving Ground, Maryland 21010 ‡ Research Microbiologist, Research Division, Department of Physiology and Immunology, US Army Medical Research Institute of Chemical Defense, 3100 Ricketts Point Road, Aberdeen Proving Ground, Maryland 21010 § Director, Division of Biochemistry, Walter Reed -

Enaminone Modulators of Extrasynaptic Α4β3δ Γ-Aminobutyric Acida Receptors Reverse Electrographic Status Epilepticus in the Rat After Acute Organophosphorus Poisoning
UC Irvine UC Irvine Previously Published Works Title Enaminone Modulators of Extrasynaptic α4β3δ γ-Aminobutyric AcidA Receptors Reverse Electrographic Status Epilepticus in the Rat After Acute Organophosphorus Poisoning. Permalink https://escholarship.org/uc/item/0bg5c30x Journal Frontiers in pharmacology, 10(MAY) ISSN 1663-9812 Authors Johnstone, Timothy BC McCarren, Hilary S Spampanato, Jay et al. Publication Date 2019 DOI 10.3389/fphar.2019.00560 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California fphar-10-00560 May 22, 2019 Time: 18:2 # 1 ORIGINAL RESEARCH published: 24 May 2019 doi: 10.3389/fphar.2019.00560 Enaminone Modulators of Extrasynaptic a4b3d g-Aminobutyric AcidA Receptors Reverse Electrographic Status Epilepticus in the Rat After Acute Organophosphorus Poisoning Timothy B. C. Johnstone1*, Hilary S. McCarren2, Jay Spampanato3, F. Edward Dudek3, John H. McDonough2, Derk Hogenkamp1 and Kelvin W. Gee1 1 Department of Pharmacology, School of Medicine, University of California, Irvine, Irvine, CA, United States, 2 Neuroscience Department, Medical Toxicology Research Division, United States Army Research Institute of Chemical Defense, Aberdeen, Edited by: MD, United States, 3 Department of Neurosurgery, University of Utah School of Medicine, Salt Lake City, UT, United States A. Leslie Morrow, The University of North Carolina at Chapel Hill, United States Seizures induced by organophosphorus nerve agent exposure become refractory -

Eyal Application No. 61/141168, Filed on Dec. ENEE
USOO9278134B2 (12) United States Patent (10) Patent No.: US 9.278,134 B2 Rogers et al. (45) Date of Patent: Mar. 8, 2016 (54) DUAL FUNCTIONING IONIC LIQUIDS AND 4,891.386 A * 1/1990 Gasparotti .................... 514,555 SALTS THEREOF 4,970,156 A 11/1990 Avrameas et al. 5,221,758 A 6/1993 Maynard 5,246,716 A 9, 1993 Sedun et al. (75) Inventors: Robin D. Rogers, Tuscaloosa, AL (US); 5,679,146 A 10/1997 Kalt et al. Daniel T. Daly, Tuscsaloosa, AL (US); 5,683,832 A 1 1/1997 Bonhote et al. Douglas MacFarlane, Victoria (AU); 5,714,536 A 2/1998 Ziolo et al. Janet L. Scott, Port Sunlight (GB); 37:6 A SE May, 1 Kenneth R. Seddon, Donaghadee (E), 5,827.602 A 10/1998 KochSC et al.ca. Gabriela Gurau, Tuscaloosa, AL (US); 5,856,513 A 1/1999 Ueet al. Katharina Bica, Vienna (AT); Jelena 6,001,342 A 12/1999 Forestier et al. Turanjanin, Victoria (AU); Pamela M. 6,376,712 B2 4/2002 Narizuka et al. Dean, Victoria (AU) 6,451,220 B1 9, 2002 Ziolo et al. s 6,613,310 B1 9/2003 Campbell et al. 6,808,557 B2 10/2004 Holbrey et al. (73) Assignees: The Board of Trustees of the 6,824,599 B2 11/2004 Swatloski et al. University of Alabama, Tuscaloosa, AL 6,967,074 B2 11/2005 Duffy et al. (US); Monash University, Melbourne 2002/0010291 A1 1/2002 Murphy (AU); Queen's University Belfast, 2003/0059604 A1 3/2003 Hattori et al. -
TRC Price List 2010
863180-05-6 A101000 A2-PE 188062-50-2 Abacavir Sulfate A105000 A105002 Abacavir-d4 384380-52-3 A105005 Abacavir Carboxylate 384329-76-4 A105010 Abacavir 5’-β-D-Glucuronide 136470-77-4 A105020 Abacavir 5’-Phosphate Abacavir 5’-(2,3,4-Tri-O-isobytyryl)-β-D-glucuronic A105030 Acid Methyl Ester 71751-41-2 A107000 Abamectin 154229-18-2 A108500 Abiraterone Acetate A108502 Abiraterone Acetate-d4 21293-29-8 A110000 (+)-cis,trans-Abscisic Acid, 98% A110002 (+)-cis,trans-Abscisic Acid-d6 192987-96-5 A110010 rac 8’-Acetylene Abscisic Acid Methyl Ester 923564-51-6 A112500 ABT 263 A112502 ABT 263-d8 852808-04-9 A112550 ABT 737 A112552 ABT 737-d8 912444-00-9 A112580 ABT 888 447407-36-5 A115600 AC 42 77337-73-6 A120000 Acamprosate Calcium A120002 Acamprosate-d12 Calcium Trihydrate A120003 Acamprosate-d6 Calcium Trihydrate 56180-94-0 A123500 Acarbose A123502 Acarbose-d4 117065-98-2 A123505 Acarbose Tridecaacetate 34381-68-5 A123800 Acebutolol Hydrochloride Acebutolol 37517-30-9 A123801 Discontinued. -

Interactions Pharmacokinetics Profile Profile Profile Adverse Effects And
Ammonium 1541 15. Bos JD, et al. Allergic contact dermatitis to amyl nitrite ('poppers'). r i has been suggested for each gram of resin. It should not be Contact Dermatitis 1985; 12: 109. P.r.�P.�_ ?l ()_n.�................. ................... given in fruit juices that have a high potassium content. 16. Foroozan M, et al. Dermatose faciale aux poppers. Ann Dermatol Venereal Proprietary Preparations (details are given in Volume B) 2009; 136: 298-9. When oral administration is difficult, calcium poly Single-ingredient Preparations, Jpn: Kremezin; Kyucal. styrene sulfonate may be given rectally as an enema. The usual daily dose is 30 g given as a suspension in 150 mL of Handling and storage. Amyl nitrite is very flammable water or glucose 10%, or IOOmL of 2% methylcellulose and must not be used where it may be ignited. It should plus 100 mL of water, and retained, if possible, for at least 9 be protected from light. Atipamezole {BAN, USAN, r/NN) hours. Initial therapy may involve both oral and rectal At tparilezoi: Arl pametoium; MPV·l248: AT•ma· routes. Following retention of the enema the colon should Pregnancy. Licensed product information warns that Atipq_m· ezoil2; Melon · be irrigated to remove the resin. maternal use of amyl nitrite during pregnancy reduces .. For doses in children, see below. blood pressure in the mother and blood flow across the placenta. Administration in children. Calcium polystyrene sulfonate is used in neonates and children to enhance potassium Interactions excretion in the treatment of hyperkalaemia associated with anuria or severe oliguria, and in dialysis patients. Use Like organic nitrates, amyl nitrite and other inhaled volatile is not recommended in neonates with reduced gut moti nitrites may potentiate the hypotensive effects of phospho Atipamezole Hydrochloride {BANM, r/NNM) lity. -
Parts 1 and 2
TOXICOLOGY INVESTIGATION INTO BINKILL GARBAGE BIN FUMIGATION PRODUCT FOR WASTE AND COMMUNITY PROTECTION DIVISION PENRITH COUNCIL Parts 1 and 2 TOXICOLOGIST: CHRIS DERRY UNIVERSITY OF WESTERN SYDNEY JUNE 2010 ABSTRACT In March 2010 the University of Western Sydney was requested by Penrith City Council to carry out a toxicological assessment of the potential use by residents of Penrith Council area of a commercial pesticide product, Binkill, with potential for exposure to residents, their families and pets. The product was to be used as recommended by the manufacturer by residents as a slow release fly killer contained in a capsule to be placed by householders in bulk collection bins for green and kitchen waste, with the intention of controlling flies and their immature forms during storage, pending collection as part of a municipal composting operation. Part 1 of this toxicology report concerns a critical review of the research literature relating to dichlorvos and naphthalene as the main active ingredients. This review was not to be merely a summary of findings in relevant literature, but an evaluation of those findings with relevance to the safe use of the product in the intended household setting, with exposition of any problems which might be envisaged from a toxicological perspective. Part 2 of the report is an original health risk assessment based on the use of Binkill itself in terms of the device which is used to contain the active ingredients, labelling of the product packaging and device, risk to Penrith Council staff or public in terms of storage, handling use and disposal of the product, and potential local threats to the environment as a consequence of using the product. -

Adverse Effects and Precautions Pharmacokinetics Uses And
Pentetic 1571 Thalassaemia. Iron overload in patients with thalassaemia Treatment may be monitored by the determination of (p. 1124.1) is usually treated with desferrioxamine, but blood-cholinesterase concentrations and clinical symptoms. auditory toxicity can result. Calcium pentetic acid has Other oximes with cholinesterase-reactivating properties been used as an alternative. A studyr in 5 patients in that have been used similarly include asoxime chloride whom desferrioxarnine had to be withdrawn because of (p. 1541.1). obidoxime chloride (p. 1566.3), and high-tone deafness found tbat the pentetate was as effec trimedoxime bromide (p. 1579.1). tive as desferrioxamine and hearing improved during treatment. Oral zinc supplements were necessary to main Administration in children. Pralidoxime chloride is used in tain adequate plasma-zinc concentrations. children as an adjunct to atropine jn the treatment of l. Wanke B, et al. Reversal of desferrioxamine induced auditory organophosphorus poisoning; the BNFC recommends the neurotoxicity during treatment with Ca-DTPA. Arch Dis Child 1989; 64: same dose as that recommended by the BNF for adults, see 77-82. above. Adverse Effects and Precautions Pharmacopoeias. In It. Carbamate poisoning.The use of pralidoxime for poison Adverse effects that have been reported with calcium or zinc ing due to carbamate insecticides is controversial. Licensed trisodium pentetate include headache and lightheadedness. Uses and Administration product information states that pralidoxime should not be Pentetates chelate trace metals and supplements may be used to treat poisoning by carbamate pesticides as it may Pralidoxime is a cholinesterase reactivator. It is used as an needed with long-term use. Trace metals and serum increase toxicity, and it has been suggested that as carba adjunct to, but not as a substitute for, atropine in the electrolytes should be monitored during use. -

Practical Guide for Medical Management of Chemical Warfare Casualties
Practical Guide for Medical Management of Chemical Warfare Casualties Practical Guide for Medical Management of Chemical Warfare Casualties OPCW Organisation for the Prohibition of Chemical Weapons International Cooperation and Assistance Division Assistance and Protection Branch 2016 Disclaimer This Guide contains information, guidelines, diagrams and other materials addressed to medical practitioners who are engaged in the treatment of casualties of chemical weapons. It is made available to the public for information purposes, but is not intended to be used by the public. All decisions regarding patient care must be made with a healthcare provider and consider the unique characteristics of each patient. The views and opinions expressed in this Guide and in the suggested reading materials are those of the authors and do not reflect the views of the OPCW or the individuals involved in the development of this Guide. These materials are cited as a service to readers and do not imply endorsement by the OPCW or the individuals involved in the development of this Guide. The OPCW or the individuals involved in the development of this Guide are not responsible for the content of third party websites. The information contained in this publication is accurate to the best of the OPCW’s knowledge; however, neither the OPCW nor the individuals involved in the development of this Guide shall be liable under any circumstances for the correctness, accuracy or comprehensiveness of such information, or for the consequences of its use. Contents Foreword