Phencyclidine Assay Metabolites, and Primarily As Unidentified Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold
Article Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold Nutcha Larpant 1, Yaneenart Suwanwong 2, Somchai Boonpangrak 3 and Wanida Laiwattanapaisal 4,5,* 1 Graduate Program in Clinical Biochemistry and Molecular Medicine, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 2 Department of Clinical Microscopy, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 3 Center for Research and Innovation, Faculty of Medical Technology, Mahidol University, Nakhon Pathom 73170, Thailand; [email protected] 4 Department of Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand 5 Electrochemistry and Optical Spectroscopy Center of Excellence, Chulalongkorn University, Bangkok 10330, Thailand * Correspondence: [email protected] Received: 22 January 2019; Accepted: 20 March 2019; Published: 26 March 2019 Abstract: Commercially available sorbent materials for solid-phase extraction are widely used in analytical laboratories. However, non-selective binding is a major obstacle for sample analysis. To overcome this problem, molecularly imprinted polymers (MIPs) were used as selective adsorbent materials prior to determining target analysts. In this study, the use of non-covalent molecularly imprinted polymers (MIPs) for cotinine adsorption on a paper-based scaffold was studied. Fiberglass paper was used as a paper scaffold for cotinine-selective MIP adsorption with the use of 0.5% agarose gel. The effects of salt, pH, sample matrix, and solvent on the cotinine adsorption and extraction process were investigated. Under optimal conditions, the adsorption isotherm of synthesized MIPs increased to 125.41 µg/g, whereas the maximum adsorption isotherm of non-imprinted polymers (NIPs) was stable at 42.86 µg/g. -

Hallucinogens - LSD, Peyote, Psilocybin, and PCP
Information for Behavioral Health Providers in Primary Care Hallucinogens - LSD, Peyote, Psilocybin, and PCP What are Hallucinogens? Hallucinogenic compounds found in some plants and mushrooms (or their extracts) have been used— mostly during religious rituals—for centuries. Almost all hallucinogens contain nitrogen and are classified as alkaloids. Many hallucinogens have chemical structures similar to those of natural neurotransmitters (e.g., acetylcholine-, serotonin-, or catecholamine-like). While the exact mechanisms by which hallucinogens exert their effects remain unclear, research suggests that these drugs work, at least partially, by temporarily interfering with neurotransmitter action or by binding to their receptor sites. This InfoFacts will discuss four common types of hallucinogens: LSD (d-lysergic acid diethylamide) is one of the most potent mood-changing chemicals. It was discovered in 1938 and is manufactured from lysergic acid, which is found in ergot, a fungus that grows on rye and other grains. Peyote is a small, spineless cactus in which the principal active ingredient is mescaline. This plant has been used by natives in northern Mexico and the southwestern United States as a part of religious ceremonies. Mescaline can also be produced through chemical synthesis. Psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine) is obtained from certain types of mushrooms that are indigenous to tropical and subtropical regions of South America, Mexico, and the United States. These mushrooms typically contain less than 0.5 percent psilocybin plus trace amounts of psilocin, another hallucinogenic substance. PCP (phencyclidine) was developed in the 1950s as an intravenous anesthetic. Its use has since been discontinued due to serious adverse effects. How Are Hallucinogens Abused? The very same characteristics that led to the incorporation of hallucinogens into ritualistic or spiritual traditions have also led to their propagation as drugs of abuse. -

Cerebellar Toxicity of Phencyclidine
The Journal of Neuroscience, March 1995, 75(3): 2097-2108 Cerebellar Toxicity of Phencyclidine Riitta N&kki, Jari Koistinaho, Frank Ft. Sharp, and Stephen M. Sagar Department of Neurology, University of California, and Veterans Affairs Medical Center, San Francisco, California 94121 Phencyclidine (PCP), clizocilpine maleate (MK801), and oth- Phencyclidine (PCP), dizocilpine maleate (MK801), and other er NMDA antagonists are toxic to neurons in the posterior NMDA receptor antagonistshave attracted increasing attention cingulate and retrosplenial cortex. To determine if addition- becauseof their therapeutic potential. These drugs have neuro- al neurons are damaged, the distribution of microglial ac- protective properties in animal studies of focal brain ischemia, tivation and 70 kDa heat shock protein (HSP70) induction where excitotoxicity is proposedto be an important mechanism was studied following the administration of PCP and of neuronal cell death (Dalkara et al., 1990; Martinez-Arizala et MK801 to rats. PCP (10-50 mg/kg) induced microglial ac- al., 1990). Moreover, NMDA antagonists decrease neuronal tivation and neuronal HSP70 mRNA and protein expression damage and dysfunction in other pathological conditions, in- in the posterior cingulate and retrosplenial cortex. In ad- cluding hypoglycemia (Nellgard and Wieloch, 1992) and pro- dition, coronal sections of the cerebellar vermis of PCP (50 longed seizures(Church and Lodge, 1990; Faingold et al., 1993). mg/kg) treated rats contained vertical stripes of activated However, NMDA antagonists are toxic to certain neuronal microglial in the molecular layer. In the sagittal plane, the populations in the brain. Olney et al. (1989) demonstratedthat microglial activation occurred in irregularly shaped patch- the noncompetitive NMDA antagonists,PCP, MK801, and ke- es, suggesting damage to Purkinje cells. -

From NMDA Receptor Hypofunction to the Dopamine Hypothesis of Schizophrenia J
REVIEW The Neuropsychopharmacology of Phencyclidine: From NMDA Receptor Hypofunction to the Dopamine Hypothesis of Schizophrenia J. David Jentsch, Ph.D., and Robert H. Roth, Ph.D. Administration of noncompetitive NMDA/glutamate effects of these drugs are discussed, especially with regard to receptor antagonists, such as phencyclidine (PCP) and differing profiles following single-dose and long-term ketamine, to humans induces a broad range of exposure. The neurochemical effects of NMDA receptor schizophrenic-like symptomatology, findings that have antagonist administration are argued to support a contributed to a hypoglutamatergic hypothesis of neurobiological hypothesis of schizophrenia, which includes schizophrenia. Moreover, a history of experimental pathophysiology within several neurotransmitter systems, investigations of the effects of these drugs in animals manifested in behavioral pathology. Future directions for suggests that NMDA receptor antagonists may model some the application of NMDA receptor antagonist models of behavioral symptoms of schizophrenia in nonhuman schizophrenia to preclinical and pathophysiological research subjects. In this review, the usefulness of PCP are offered. [Neuropsychopharmacology 20:201–225, administration as a potential animal model of schizophrenia 1999] © 1999 American College of is considered. To support the contention that NMDA Neuropsychopharmacology. Published by Elsevier receptor antagonist administration represents a viable Science Inc. model of schizophrenia, the behavioral and neurobiological KEY WORDS: Ketamine; Phencyclidine; Psychotomimetic; widely from the administration of purportedly psychot- Memory; Catecholamine; Schizophrenia; Prefrontal cortex; omimetic drugs (Snyder 1988; Javitt and Zukin 1991; Cognition; Dopamine; Glutamate Jentsch et al. 1998a), to perinatal insults (Lipska et al. Biological psychiatric research has seen the develop- 1993; El-Khodor and Boksa 1997; Moore and Grace ment of many putative animal models of schizophrenia. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Effect of Varenicline Combined with Medical Management on Alcohol Use Disorder with Comorbid Cigarette Smoking
Table of Contents I. Summary of Changes to the Statistical Plan …………………. 2. II. Summary of Changes to the Protocol………………………… 2. III. Original Protocol at Trial Initiation ………………………… 5. 1 Downloaded From: https://jamanetwork.com/ on 09/28/2021 I. Summary of changes to the statistical analysis plan The original statistical analysis plan is described in the original protocol below beginning on page 20. In the original statistical analysis plan the stratification factor sex was omitted unintentionally from the primary model specification for the main outcome (percent heavy drinking days, PHDD) although sex was identified as a potential moderator in an exploratory aim. At the analysis stage, we included both stratification factors (site and sex) and their interactions with treatment in the model for PHDD consistent with prior hypothesis that men and women differ in their smoking and drinking behavior and treatment response, and with good statistical practice. Baseline percent heavy drinking days was specified as a covariate in the original analysis plan but in response to comments by the statistical reviewer was dropped as a covariate and included in the final mixed model analyses reported in the paper. In order to make the baseline and end-point measures comparable, baseline percent heavy drinking days and end-point percent heavy drinking days were both summarized over 8 week periods. This 8-week duration was pre-planned for the end-point measure but modified from the original intention to use 30 days of the baseline period. Unstructured variance-covariance matrix was used for the errors since variances at baseline and end-point were different. -

OPRM1 Genetic Polymorphisms Are Associated with the Plasma Nicotine Metabolite Cotinine Concentration in Methadone Maintenance Patients: a Cross Sectional Study
Journal of Human Genetics (2013) 58, 84–90 & 2013 The Japan Society of Human Genetics All rights reserved 1434-5161/13 www.nature.com/jhg ORIGINAL ARTICLE OPRM1 genetic polymorphisms are associated with the plasma nicotine metabolite cotinine concentration in methadone maintenance patients: a cross sectional study Yu-Ting Chen1, Hsiao-Hui Tsou2, Hsiang-Wei Kuo1, Chiu-Ping Fang1, Sheng-Chang Wang1, Ing-Kang Ho1,3,4, Yao-Sheng Chang1, Chia-Hui Chen1, Chin-Fu Hsiao2, Hsiao-Yu Wu2, Keh-Ming Lin1, Andrew CH Chen5, Jyy-Jih Tsai-Wu6 and Yu-Li Liu1,7,8 Majority of the heroin-dependent patients smoke cigarettes. Although it has been reported that the OPRM1 genetic polymorphism is associated with the brain mu-opioid receptor binding potential in cigarette smokers, there is no direct evidence showing the impact of plasma cotinine, a nicotine metabolite, on treatment responses to methadone maintenance treatment (MMT). In this study, we aimed to test the hypothesis that the genetic polymorphisms in the OPRM1 are associated with the methadone treatment responses and the severity of cigarette smoking directly measured by the plasma concentration of cotinine in a Taiwanese MMT cohort. Fifteen OPRM1 single-nucleotide polymorphisms (SNPs) were selected and genotyped on DNA samples of 366 MMT patients. Plasma concentrations of cotinine were measured by cotinine enzyme-linked immunosorbent assay. The plasma cotinine concentration had positive correlation with concentrations of methadone (P ¼ 0.042) and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenyl-pyrrolidine (P ¼ 0.037). Methadone treatment non-responders, defined by a positive urine morphine test, had a higher plasma concentration of cotinine (P ¼ 0.005), but a lower plasma concentration-to- dose ratio of both R- and S-methadone (P ¼ 0.001 and 0.012, respectively) than the responders. -

Hallucinogens & Dissociative Drugs
® DRUG FACT SHEET Hallucinogens & Dissociative Drugs Some effects of PCP including depression and memory loss may last six months to a year following prolonged daily use. Class of drug: Hallucinogens (most common form is LSD) Dissociative drugs (most commonly form is PCP) Main active ingredient: Hallucinogens: Lysergic acid diethylamide, mescaline, psilocybin, ibogaine Dissociative: Phencyclidine What it looks like: LSD: Clear, odorless liquid, brightly colored tablets, impregnated blotter paper, thin squares of gelatin PCP: liquid, capsules, white crystalline powder, gum There are hundreds of synthetic hallu - Street names: Lysergic acid diethylamide: LSD, Acid, Blotter, cinogens on the market today including Phencyclidine: PCP, Angel Dust, Loveboat, Wack 25I-NBOMe (N-Bomb) and 2C-I (Smiles) which have been attributed to How it is used: Both hallucinogens and dissociative drugs can be multiple deaths and significant injuries. swallowed, injected or smoked. LSD liquid and gelatin They are generally found as powders, liq - forms can be put in the eyes. PCP is often sprinkled uids, soaked into blotter paper or laced on or sprayed on cigarettes, parsley and marijuana. something edible. Both drugs are classi - fied as Schedule I substances, making Duration of high: Hallucinogens: effects begin within 30 to 90 minutes possession, distribution and manufacture and last from six to twelve hours illegal. PCP: effects begin within minutes and last for hours Withdrawal symptoms: Depression, memory loss U.S. information Effects: Physical (both) —increased heart rate and blood pressure, elevated body temperature, loss of In 2014, nearly 1.3 million appetite, loss of muscle coordination, slurred speech Americans aged 12 and older Hallucinogens reported using LSD in the past Mental —hallucinations; intensified senses; distortion year and 90,000 reported using of time, reality and environment; confusion; mood PCP in the past year. -

Nicotine Metabolism and CYP2D6 Phenotype in Smokers
Vol. 10, 261–263, March 2001 Cancer Epidemiology, Biomarkers & Prevention 261 Short Communication Nicotine Metabolism and CYP2D6 Phenotype in Smokers Neil E. Caporaso,1 Caryn Lerman, Janet Audrain, CYP2D6 (debrisoquine hydroxylase) has been studied as a Neil R. Boyd, David Main, Haleem J. Issaq, putative lung cancer susceptibility factor, and it has been pro- Bill Utermahlan, Roni T. Falk, and Peter Shields posed that this enzyme may contribute to the metabolism of Division of Cancer Epidemiology and Genetics, National Cancer Institute, nicotine, thereby influencing the amount a person smokes and Bethesda, Maryland 20892 [N. E. C., R. T. F.]; Lombardi Cancer Research the delivery of carcinogens. Some in vitro (1) and population Center, Georgetown University, Washington, D.C. 20007 [C. L., J. A., (2) studies have suggested a role for CYP2D6 in nicotine N. R. B., D. M.]; Laboratory of Chemical Synthesis and Analysis, Frederick metabolism, although recent work indicates CYP2A6 is the Cancer Research Center, Frederick, Maryland 21701 [H. J. I., B. U.]; and Laboratory of Human Carcinogenesis, Division of Cancer Etiology, NIH, most important P-450 in nicotine metabolism (3, 4). The National Cancer Institute, Bethesda, Maryland 20892 [P. S.] CYP2D6 MP2 is determined by administering DM (5) and determining the ratio of urinary metabolites. A low ratio of unchanged drug:metabolite identifies extensive metabolizers, Abstract whereas PMs exhibit a high ratio. The hypothesis that a poly- We tested the hypothesis that the polymorphic enzyme morphic gene influences nicotine disposition is important be- CYP2D6 is related to nicotine metabolism in 261 healthy cause addicted smokers engage in smoking behavior in such a subjects enrolling in a smoking cessation clinic. -

Phencyclidine: an Update
Phencyclidine: An Update U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES • Public Health Service • Alcohol, Drug Abuse and Mental Health Administration Phencyclidine: An Update Editor: Doris H. Clouet, Ph.D. Division of Preclinical Research National Institute on Drug Abuse and New York State Division of Substance Abuse Services NIDA Research Monograph 64 1986 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Alcohol, Drug Abuse, and Mental Health Administratlon National Institute on Drug Abuse 5600 Fishers Lane Rockville, Maryland 20657 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, DC 20402 NIDA Research Monographs are prepared by the research divisions of the National lnstitute on Drug Abuse and published by its Office of Science The primary objective of the series is to provide critical reviews of research problem areas and techniques, the content of state-of-the-art conferences, and integrative research reviews. its dual publication emphasis is rapid and targeted dissemination to the scientific and professional community. Editorial Advisors MARTIN W. ADLER, Ph.D. SIDNEY COHEN, M.D. Temple University School of Medicine Los Angeles, California Philadelphia, Pennsylvania SYDNEY ARCHER, Ph.D. MARY L. JACOBSON Rensselaer Polytechnic lnstitute National Federation of Parents for Troy, New York Drug Free Youth RICHARD E. BELLEVILLE, Ph.D. Omaha, Nebraska NB Associates, Health Sciences Rockville, Maryland REESE T. JONES, M.D. KARST J. BESTEMAN Langley Porter Neuropsychiatric lnstitute Alcohol and Drug Problems Association San Francisco, California of North America Washington, D.C. DENISE KANDEL, Ph.D GILBERT J. BOTV N, Ph.D. College of Physicians and Surgeons of Cornell University Medical College Columbia University New York, New York New York, New York JOSEPH V. -
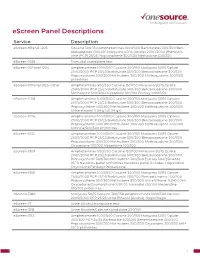
Escreen Panel Descriptions
eScreen Panel Descriptions Service Description eScreen-9Panel-1205 Cocaine 300/150 Amphetamines 1000/500 Barbiturates 300/300 Ben- zodiazepines 300/300 Marijuana 50/15 Opiates 2000/2000 Phencycli- dine (PCP) 25/25 Propoxyphene 300/300 Methadone 300/300 eScreen-1065 Tramadol standalone test eScreen-10Panel-1204 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 pH/nitrites eScreen-10Panel-3125-Other Amphetamines 500/250 Cocaine 150/100 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Methadone 300/300 Oxycodone 100/100 Ecstasy 1000/250 eScreen-1208 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiates 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Urine ethanol 0.04 g%/0.04 g% eScreen-1224 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiates 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Cotinine 500/500 pH/nitrites eScreen-1232 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Oxycodone 100/100 Meperidine 100/100 eScreen-1309 Amphetamines 500/250 Cocaine 150/100 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone -

Chronic Smokeless Tobacco Consumption Contributes to the Development of Renal Diseases in the Human Male Volunteers
Journal of Analytical & Pharmaceutical Research Research Article Open Access Chronic smokeless tobacco consumption contributes to the development of renal diseases in the human male volunteers Abstract Volume 7 Issue 6 - 2018 Smokeless tobacco may able to induce microalbuminuria. The present study S Fareeda Begum,1 G Nagajothi,2 K investigates the relation between nicotine and cotinine with renal function in gutkha Swarnalatha,1 C Suresh Kumar,1 Narendra and khaini users. Methods: The levels of nicotine and cotinine were estimated by HPLC 1 methods and other urine variables were detected by spectrophotometric methods. Maddu 1 Current smokeless tobacco users have shown that significantly elevated levels of Department of Biochemistry, Sri Krishnadevaraya University, India nicotine, cotinine, and epinephrine excretion in the urine than non-tobacco users. 2Department of Corporate Secretaryship, Queen Mary’s Renal function was assessed by glomerular filtration rate (GFR), levels of urea, and College (Autonomous), India creatinine. Among the kidney function measures that we examined, microalbuminuria, decreased glomerular filtration rate, and creatinine clearance were found associated Correspondence: Narendra Maddu, Assistant Professor, with gutkha and khaini users. Significantly decreased proteinuria, urea and increased Department of Biochemistry, Sri Krishnadevaraya University, levels of uric acid and creatinine excretion with the concomitant increase in plasma Ananthapuramu-515003, Andhra Pradesh, India, Tel 91+ total proteins, urea, and decreased uric acid levels were observed in the group I and 9441983797, Email group II users compared to group III users. The products of smokeless tobacco are regarded as good predictors of assessing the free radical levels in the cells. The active Received: June 01, 2018 | Published: November 26, 2018 markers of nitroxidative stress have been elevated progressively with the uptake of nicotine and exposure.