Duke University Dissertation Template
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
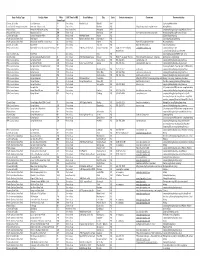
Sorted by Facility Type.Xlsm
Basic Facility Type Facility Name Miles AVG Time In HRS Street Address City State Contact information Comments Known activities (from Cary) Comercial Facility Ace Adventures 267 5 hrs or less Minden Road Oak Hill WV Kayaking/White Water East Coast Greenway Association American Tobacco Trail 25 1 hr or less Durham NC http://triangletrails.org/american- Biking/hiking Military Bases Annapolis Military Academy 410 more than 6 hrs Annapolis MD camping/hiking/backpacking/Military History National Park Service Appalachian Trail 200 5 hrs or less Damascus VA Various trail and entry/exit points Backpacking/Hiking/Mountain Biking Comercial Facility Aurora Phosphate Mine 150 4 hrs or less 400 Main Street Aurora NC SCUBA/Fossil Hunting North Carolina State Park Bear Island 142 3 hrs or less Hammocks Beach Road Swannsboro NC Canoeing/Kayaking/fishing North Carolina State Park Beaverdam State Recreation Area 31 1 hr or less Butner NC Part of Falls Lake State Park Mountain Biking Comercial Facility Black River 90 2 hrs or less Teachey NC Black River Canoeing Canoeing/Kayaking BSA Council camps Blue Ridge Scout Reservation-Powhatan 196 4 hrs or less 2600 Max Creek Road Hiwassee (24347) VA (540) 777-7963 (Shirley [email protected] camping/hiking/copes Neiderhiser) course/climbing/biking/archery/BB City / County Parks Bond Park 5 1 hr or less Cary NC Canoeing/Kayaking/COPE/High ropes Church Camp Camp Agape (Lutheran Church) 45 1 hr or less 1369 Tyler Dewar Lane Duncan NC Randy Youngquist-Thurow Must call well in advance to schedule Archery/canoeing/hiking/ -

North Carolina's State Parks: Disregarded and in Disrepair
North Carolina's State Parks: Disregarded and in Disrepair By Bill Krueger and Mike McLaughlin More than seven million people visit North Carolina's state parks and recreation areas each year-solid evidence that the public supports its state park system. But for years, North Carolina has routinely shown up at or near the bottom in funding for parks, and its per capita operating budget currently ranks 49th in the nation. Some parks are yet to be opened to the public due to lack of facilities, and parts of other parks are closed because existing facilities are in a woeful state of disrepair. Indeed, parks officials have identified more than $113 million in capital and repair needs, nearly twice as much as has been spent on the parks in the system's 73-year history. Just recently, the state has begun making a few more gestures toward improving park spending. But the question remains: Will the state commit the resources needed to overcome decades of neglect? patrol two separate sections of the park, pick up highway in the narrowing strip of unde- trash, clean restrooms and bathhouses, and main- veloped property that separates the bus- tain dozens of deteriorating buildings . "I've got a Wedgedtling citiesbetween of Raleigh aninterstate and Durhamanda major lies a total of 166 buildings - most of them built between refuge from commercialization called William B. 1933 and 1943," says Littrell. "I've got buildings Umstead State Park. with five generations of patches- places where The 5,400-acre oasis has become an easy re- patches were put on the patches that were holding treat to nature in the midst of booming growth. -
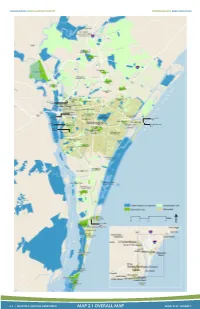
Map 2.1 Overall Map Move
WILMINGTON/NEW HANOVER COUNTY COMPREHENSIVE GREENWAY PLAN Northside Park Robert Strange Greensboro Park Park Optimist Johnnie Mercers Park Pier Legion Stadium Masonboro Island Coastal Resreve Snows Cut Park 2-5 | CHAPTER 2: EXISTING CONDITIONS MAP 2.1 OVERALL MAP MOVE. PLAY. CONNECT. WILMINGTON/NEW HANOVER COUNTY COMPREHENSIVE GREENWAY PLAN This map displays some highlights from comments collected during steering committee meetings that took place in early 2012. GE Wilmington employs more than 2,000 people in its Castle Hayne facility1. The University of North Carolina at Wilmington employs over 1,800 faculty and staff, teaching over 2 The Riverwalk is a scenic 13,000 students each year . boardwalk along the east bank of the Cape Fear River in downtown Wilmington. It provides a place to stroll along the water minutes from the buildings, restaurants, and Middle Sound Loop Rd. is a shops forming the heart of popular on-road recreation downtown. destination. The Forest Hills Loop is a popular walking and jogging destination just east of downtown. The loop is approximately three miles in length and passes by the Wilmington “The Loop” around Wrightsville Beach YMCA and Beaumont Park. is a popular walking and jogging destination near the ocean. The Loop is formed by a concrete sidewalk varying in width from 5’ to 10’ and passes by scenic views of the sound, as well as New Hanover Regional Medical by the shops and restaurants forming Center is a major economic driver the commercial district along Lumina in the region, with more than 4,000 Avenue. employees3. The shopping center at the junction of College Rd. -

Checklist of the Fishes Documented from the Zeke's Island And
Technical Report Series 2002: 2 Checklist of the Fishes Documented from the Zeke’s Island and Masonboro Island Components of the North Carolina National Estuarine Research Reserve Steve W. Ross and John Bichy November 2002 ABOUT THIS DOCUMENT The North Carolina National Estuarine Research Reserve is conducting basic biological inventories of the biota in and near the four reserve components. This checklist of fishes in two Reserve components represents the first major product in that area. We intend that this base line data will document the Reserve’s ichthyofauna and will also serve as a benchmark to measure future changes. OBTAINING COPIES This document is available for downloading as a PDF from http://www.ocrm.nos.noaa.gov/nerr/resource.html HOW TO CITE THIS DOCUMENT The appropriate citation for this document is: Ross, S.W. and J. Bichy. 2002. Checklist of the Fishes Documented from the Zeke’s Island and Masonboro Island Components of the North Carolina National Estuarine Research Reserve. National Estuarine Research Reserve Technical Report Series 2002: 2. CONTACT INFORMATION FOR THE AUTHORS Dr. Steve Ross, North Carolina National Estuarine Research Reserve, 5600 Marvin Moss Ln. Wilmington, NC 28409; [email protected] John Bichy, Chesapeake Biological Laboratory, 1 Williams Street, Solomons, MD 20688, [email protected] DISCLAIMER The contents of this report do not necessarily reflect the views or policies of the Estuarine Reserves Division or the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, in any advertising or sales promotion, which would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has as its purpose an interest to cause directly or indirectly the advertised product to be used or purchased because of this publication. -

Piping Plovers in North Carolina
Compilation and Assessment of Piping Plover Wintering and Migratory Staging Area Data in North Carolina Susan Cameron and David Allen NC Wildlife Resources Commission, NC Marcia Lyons Cape Hatteras National Seashore, NC Jeff Cordes Cape Lookout National Seashore, NC Sidney Maddock Buxton, NC Piping Plovers in North Carolina • North Carolina is unique because piping plovers can be observed twelve months out of the year • North Carolina is at the northern extent of the wintering range and is an important stopover area during spring and fall migration • All three populations are known to use our coastline during the non-breeding season 1 Threats - Development/Beach Stabilization S. Maddock S. Maddock Threats – Chronic Human Disturbance 2 History of Non-breeding Piping Plover Program • In the past, surveys for migrating and wintering piping plovers were conducted mostly in an opportunistic fashion with data stored in multiple formats • In 2001, NCWRC obtained a grant from USFWS to create an Access database for non-breeding piping plover observations • Observations were compiled in an effort to identify some of the most important areas for piping plovers History of Non-breeding Piping Plover Program • In recent years, systematic surveys have been conducted at various locations including Cape Hatteras and Cape Lookout National Seashores and at several sites in association with beach stabilization projects • An observation form has been created and distributed in an effort to increase the number of sightings reported 3 Non-breeding Piping Plover -
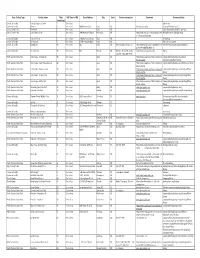
Sorted by Miles from Cary.Xlsm
Basic Facility Type Facility Name Miles AVG Time In HRS Street Address City State Contact information Comments Known activities (from Cary) Comercial Facility Triangle Aquatics Center 2 1 hr or less Swimming Comercial Facility Glenaire 4 1 hr or less 400 Glenaire Circle Cary NC Good pack activity Singing Christmas carols City / County Parks Bond Park 5 1 hr or less Cary NC Canoeing/Kayaking/COPE/High ropes City / County Parks Lake Crabtree Park 5 1 hr or less 1400 Aviation Parkway Morrisville NC http://www.wakegov.com/parks/lakecrab Biking/Mountain Biking/boating tree/Pages/default.aspx Comercial Facility Polar Ice House 5 1 hr or less 1410 Buck Jones Road Cary NC Ice skating Comercial Facility RU A Gamer 5 1 hr or less 218 Nottingham Drive Cary NC Video Arcade Games Comercial Facility Oddfellows 10 1 hr or less RDU Cary NC [email protected] http://www.rtpnet.org/troop200/forms/R Primitive Camping/Backpacking/Biking DU-CAMP-ODDFELLOWS.doc Comercial Facility Young Eagles 10 1 hr or less RDU Raleigh NC Raleigh - Richard Netherby - http://www.youngeagles.org/ Flying EAA 879 (919) 608-2316 North Carolina State Park Jordan Lake - Crosswinds 10 1 hr or less Apex NC http://www.ncparks.gov/Visit/parks/jord/ camping/hiking/backpacking/board directions.php sailing/boating/Water Skiing North Carolina State Park Jordan Lake - New Hope Overlook 10 1 hr or less Apex NC http://www.ncparks.gov/Visit/parks/jord/ camping/hiking/backpacking/Primitive camping directions.php North Carolina State Park Jordan Lake - Parkers Creek 10 1 hr or less Apex NC -

The Influence of Inlet Modifications, Geologic Framework, and Storms on the Recent Evolution of Masonboro Island, Nc
THE INFLUENCE OF INLET MODIFICATIONS, GEOLOGIC FRAMEWORK, AND STORMS ON THE RECENT EVOLUTION OF MASONBORO ISLAND, NC S. David Doughty A Thesis Submitted to the University of North Carolina at Wilmington in Partial Fulfillment Of the Requirements for the Degree of Master of Science Department of Earth Sciences University of North Carolina at Wilmington 2006 Approved by Advisory Committee ______________________________ ______________________________ ______________________________ Chair Accepted by ______________________________ Dean, Graduate School This thesis has been prepared in a style and format consistent with The Journal of Coastal Research ii TABLE OF CONTENTS ABSTRACT.........................................................................................................................v ACKNOWLEDGEMENTS.............................................................................................. vii LIST OF TABLES........................................................................................................... viii LIST OF FIGURES ........................................................................................................... ix INTRODUCTION ...............................................................................................................1 Study Area and Background ................................................................................................1 Study Area ......................................................................................................................1 Local -

NC Wetlands Passport
OLINA — AR C H T R O N — N I A L P L A T S A O C — T N M O O M UN D TAINS—PIE www.ncwetlands.org NCwetlands.org is an outreach project of the North Carolina Division of Water Resources, with funding from the US Environmental Protection Agency. Passport created in 2018. We are excited that you are joining us on a North Carolina wetland journey! We hope this passport will help you find wetlands you’ve never seen before or help you reconnect with wetlands you may have forgotten. This passport will unlock the door to your future wetland adventures in North Carolina - in wetlands right next door or wetlands on the opposite side of the state. Let your North Carolina wetland adventure begin! What is in the passport: Be safe: This passport lists and maps Have fun on your wetland over 220 publicly accessible adventures, but also be safe. When wetlands across North Carolina. possible, contact the location to Sites are listed alphabetically by confirm hours of operation. While ecoregion (Mountain, Piedmont, these wetlands are publicly and Coastal Plain) with address accessible, there may be guidelines information provided. Wetland and restrictions at some of the sites. sites are numbered in the order Be sure to follow all guidelines and listed and shown on a centerfold signage at a wetland site. map (pages 6-7). Many of these wetlands are located To find a wetland site: on state game lands which are open Use the centerfold map to find a to hunting and gates may be closed site number in an area you want to during parts of the year. -

WILDLIFE FEDERATION Jjoowildu Ulives Rwildr Npnlaces a a Summerll 2020
North Carolina WILDLIFE FEDERATION JJooWILDu uLIVES rWILDr nPnLACES a a Summerll 2020 BIG WINS FOR YOUR LAND Great news—and more challenges— for North Carolina’s public lands. FIXING THE MARINE MESS GOT BISON? pathways in conservation BY TIM GESTWICKI, NCWF CEO The Road Never Traveled enjoy a good trail. Whether in a park or wildlife refuge, trails provide a well-thought-out pathway towards I a destination, be it a birding spot or hunting location. And a good trail often avoids dangerous traverses or sketchy river crossing. Admittedly, I have strayed off many paths and trails in my life, especially in my early exploring years. The thrill of going off-road with my buddies to hike and find a remote campsite or fishing hole was exciting and fun. We were young thrill seekers and, of course, we knew everything. Problems arose, however, when we lost our way. Not scary lost, but off course so we came out of the woods in the dark and miles away from home. Sometimes that necessitated a phone call from a stranger’s house for a rescue ride home, or even resorting to hitching a ride from a willing stranger on some country road. We also often encountered unforeseen challenges along the way such as bad weather, lack of provisions during a longer-than-expected romp, or an unhappy encounter with yellow jackets. But we were young and strong- headed. We pushed through. Some of those lessons have come in handy of late. COVID-19 has changed how the world operates, and it’s clear this will take our conservation efforts down an unfamiliar—and unmarked—path. -

World War II Heritage Guide Map Rented Rooms to Soldiers and War Workers, and Blackouts
outs and dim-outs. German U-boats marauding and a crime wave, and apply equal justice. W IlMINgtoN at War offshore spilled debris from sunken Allied ships Citizens became volunteer airplane spotters, Southeastern North Carolina was a mighty on favorite bathing beaches. Until 1944, the Inland Waterway boat patrollers, air raid war- contributor to the U.S. war effort in World War orld ar threat remained of German attack by sea and air. dens and auxiliary police and firemen. Women W W II II. Wilmington was called “The Defense Capital The community coped with a dire housing short- excelled in the Aircraft Warning Service, war of the State” and became the country’s unique age, and strains on schools, transportation, med- wartime boomtown. The once-quiet city, geo- bond and scrap drives, and as Red Cross nurse's ical and social services, law enforcement, food aides. All of this was undertaken into 1944 graphically isolated for decades, suddenly found supply, security and entertainment. Families erItage through continuing low-light dim-outs and beach H itself an exploding center of military life and World War II Heritage Guide Map rented rooms to soldiers and war workers, and blackouts. defense production. historic houses were cut up into apartments. Each branch of the armed forces stationed Racially segregated entertainment and social of and The population more than doubled with the life proceeded, despite rationing of gasoline, Wilmington thousands in the area ---- the Army Air Forces at influx of military personnel, war workers and uIde ap tires, sugar, coffee, and whiskey. Front Street, Southeastern North Carolina g M the airport, the Army at Camp Davis and Fort their families. -

Bicycle Is a Vehicle and You Are Its Driver
Laws & Safety Tips Wilmington, North Carolina Metropolitan Area Bike = Vehicle Basic Traffic Riding Techniques In North Carolina, your bicycle is a vehicle and you are its driver. You share the rights and the duties with all other drivers as you use the State's Riding confidently and skillfully in traffic takes practice and an Destination and position roadway network. understanding of some basic ideas. One of the most important ideas is road position. Just where you ride on the roadway depends on several Near intersections, it’s a good idea to let your road position tell others important things: your speed, the width and condition of the road, and where you’re going. your destination. Follow The Road Rules 1 To turn right, move towards the right edge of the roadway. Bicycle 1. Ride on the right side of the road, Speed and road position 2 To go straight, keep at least three feet from the curb and stay out of going with the flow of traffic. right turn lanes. Map The closer you go to the speed of traffic, the closer you should ride To turn left, ride about three feet right of the center line or, if there to that traffic. 3 is one, use the left turn lane. 2. Obey all traffic controls — like stop signs, traffic lights, and one-way signs. 1 When everyone else is going a lot faster than you, keep well to the right. 2 When they’re going a little faster, ride near traffic. This encourages Right Left 3. Signal whenever you intend to turn, right-turners to slow and wait instead of passing at the last moment merge to another road position, or stop. -

Wilmington and Beaches-Transcending Coastal
Media Contact: Connie Nelson 866.266.9690 [email protected] Wilmington, North Carolina and its island beaches is Get Educated a year-round destination that transcends coastal visitor During WWII, the Battleship NORTH CAROLINA participated experiences. Take a stroll on the scenic Riverwalk or through in every major naval offensive in the Pacific. Before or after historic downtown, and you’ll find yourself surrounded by your tour, take in spectacular river views while walking the breweries, music, unique eats, local culture and unrivaled new, half-mile SECU Memorial Walkway surrounding the adventures around every corner. It’s just a short drive from ship. For history or science lovers, the Cape Fear Museum the award-winning riverfront to the oceanfront through recently announced its official designation as a Smithsonian Wilmington’s thriving midtown, where you’ll find parks, Affiliate. The Museum also recently unveiled its new mobile gardens, museums, a water park and city amenities. Carolina, Science Cycle program, which inspires kids to be curious, Kure and Wrightsville Beaches are well known for their think big and experiment by bringing science and hands-on beloved coastlines, but there is much more to be discovered activities to them in outdoor settings. there from farm- and sea-to-table dining to nightlife to live music. Named one of TripAdvisor’s 2018 Travelers’ Choice Beaches Abuzz “Top Destinations on the Rise,” Wilmington and Island The Town of Carolina Beach will host guided tours of the Beaches is the perfect place to stray off course for a coastal historic Carolina Beach Boardwalk June through August.