Pitfalls in the Staging Squamous Cell Carcinoma of the Hypopharynx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metformin Increases Survival in Hypopharyngeal Cancer Patients with Diabetes Mellitus: Retrospective Cohort Study and Cell-Based Analysis
pharmaceuticals Article Metformin Increases Survival in Hypopharyngeal Cancer Patients with Diabetes Mellitus: Retrospective Cohort Study and Cell-Based Analysis Yung-An Tsou 1,2, Wei-Chao Chang 3, Chia-Der Lin 1, Ro-Lin Chang 2, Ming-Hsui Tsai 1, Liang-Chun Shih 1,3, Theresa Staniczek 4 , Tsu-Fang Wu 1, Hui-Ying Hsu 3, Wen-Dien Chang 5 , Chih-Ho Lai 6,7,8,9,* and Chuan-Mu Chen 2,10,* 1 Department of Otolaryngology-Head and Neck Surgery, China Medical University Hospital, Taichung 406, Taiwan; [email protected] (Y.-A.T.); [email protected] (C.-D.L.); [email protected] (M.-H.T.); [email protected] (L.-C.S.); [email protected] (T.-F.W.) 2 Department of Life Sciences, Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung 402, Taiwan; [email protected] 3 Center for Molecular Medicine, Graduate Institute of Biomedical Sciences, China Medical University, Taichung 406, Taiwan; [email protected] (W.-C.C.); [email protected] (H.-Y.H.) 4 Department of Dermatology, Venereology and Allergology, University Medical Center and Medical Faculty Mannheim, Center of Excellence in Dermatology, Heidelberg University, 68167 Mannheim, Germany; [email protected] 5 Department of Sport Performance, National Taiwan University of Sport, Taichung 404, Taiwan; Citation: Tsou, Y.-A.; Chang, W.-C.; [email protected] 6 Department of Microbiology and Immunology, Graduate Institute of Biomedical Sciences, Lin, C.-D.; Chang, R.-L.; Tsai, M.-H.; College of Medicine, Chang Gung University, Taoyuan 333, Taiwan Shih, L.-C.; Staniczek, T.; Wu, T.-F.; 7 Department of Pediatrics, Molecular Infectious Disease Research Center, Chang Gung Memorial Hospital, Hsu, H.-Y.; Chang, W.-D.; et al. -

Early Response to Chemotherapy in Hypopharyngeal Cancer: Assessment with 11C- Methionine PET, Correlation with Morphologic Response, and Clinical Outcome
Early Response to Chemotherapy in Hypopharyngeal Cancer: Assessment with 11C- Methionine PET, Correlation with Morphologic Response, and Clinical Outcome Eric Chesnay, MD1; Emmanuel Babin, MD2; Jean Marc Constans, MD3; Denis Agostini, MD, PhD1; Arnaud Bequignon, MD2; Armelle Regeasse, MSc4; Franck Sobrio, MSc5; and Sylvain Moreau, MD2–6 1Department of Nuclear Medicine, University Hospital, Caen, France; 2Department of Head and Neck Surgery, University Hospital, Caen, France; 3Department of Radiology, University Hospital, Caen, France; 4Department of Medical Informatics and Epidemiology, University Hospital, Caen, France; 5Cyceron PET Center, Commissariat a` l’Energie Atomique/Direction des Sciences du Vivant, Caen, France; and 6Laboratory of Anatomy, University Hospital, Caen, France physicians in treatment planning by avoiding unnecessary che- Neoadjuvant chemotherapy in hypopharyngeal cancer globally motherapy courses for nonresponding patients. improves survival, but some patients do not respond to chemo- Key Words: PET; 11C-methionine; chemotherapy monitoring; therapy and adjuvant therapy is delayed. Prediction of response hypopharyngeal cancer to chemotherapy may allow physicians to optimize planned J Nucl Med 2003; 44:526–532 treatment. The aim of this study was to compare treatment response assessed early with 11C-methionine PET and morpho- logic response assessed after treatment completion with MRI. Methods: Thirteen patients with previously untreated squa- mous cell carcinoma of the hypopharynx, T3 or T4, were in- Chemotherapy and radiotherapy have made avoiding or cluded. All patients received 3 courses of chemotherapy com- delaying surgery in hypopharyngeal cancer possible. How- prising cisplatin and 5-fluorouracil. 11C-Methionine PET was ever, if improvement in the quality of life has been demon- performed before and after the first course of chemotherapy. -

Human Anatomy As Related to Tumor Formation Book Four
SEER Program Self Instructional Manual for Cancer Registrars Human Anatomy as Related to Tumor Formation Book Four Second Edition U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutesof Health SEER PROGRAM SELF-INSTRUCTIONAL MANUAL FOR CANCER REGISTRARS Book 4 - Human Anatomy as Related to Tumor Formation Second Edition Prepared by: SEER Program Cancer Statistics Branch National Cancer Institute Editor in Chief: Evelyn M. Shambaugh, M.A., CTR Cancer Statistics Branch National Cancer Institute Assisted by Self-Instructional Manual Committee: Dr. Robert F. Ryan, Emeritus Professor of Surgery Tulane University School of Medicine New Orleans, Louisiana Mildred A. Weiss Los Angeles, California Mary A. Kruse Bethesda, Maryland Jean Cicero, ART, CTR Health Data Systems Professional Services Riverdale, Maryland Pat Kenny Medical Illustrator for Division of Research Services National Institutes of Health CONTENTS BOOK 4: HUMAN ANATOMY AS RELATED TO TUMOR FORMATION Page Section A--Objectives and Content of Book 4 ............................... 1 Section B--Terms Used to Indicate Body Location and Position .................. 5 Section C--The Integumentary System ..................................... 19 Section D--The Lymphatic System ....................................... 51 Section E--The Cardiovascular System ..................................... 97 Section F--The Respiratory System ....................................... 129 Section G--The Digestive System ......................................... 163 Section -

Hypopharyngeal Cancer
Hypopharyngeal cancer The name ‘Beyond Five’ refers to the long-term support that patients with head and neck cancer often need, which often extends beyond five years after diagnosis. CONTENTS What is the hypopharynx? .................................................................................................................................. 2 What does the hypopharynx do? ........................................................................................................................ 3 What is hypopharyngeal cancer? ........................................................................................................................ 3 What causes hypopharyngeal cancer? ................................................................................................................ 3 What are the signs and symptoms of hypopharyngeal cancer? ......................................................................... 4 How is hypopharyngeal cancer diagnosed? ........................................................................................................ 5 The cancer care team .......................................................................................................................................... 7 What is staging and grading? .............................................................................................................................. 8 Treatment options for hypopharyngeal cancer ................................................................................................ 10 Surgery -

6. the Pharynx the Pharynx, Which Forms the Upper Part of the Digestive Tract, Consists of Three Parts: the Nasopharynx, the Oropharynx and the Laryngopharynx
6. The Pharynx The pharynx, which forms the upper part of the digestive tract, consists of three parts: the nasopharynx, the oropharynx and the laryngopharynx. The principle object of this dissection is to observe the pharyngeal constrictors that form the back wall of the vocal tract. Because the cadaver is lying face down, we will consider these muscles from the back. Figure 6.1 shows their location. stylopharyngeus suuperior phayngeal constrictor mandible medial hyoid bone phayngeal constrictor inferior phayngeal constrictor Figure 6.1. Posterior view of the muscles of the pharynx. Each of the three pharyngeal constrictors has a left and right part that interdigitate (join in fingerlike branches) in the midline, forming a raphe, or union. This raphe forms the back wall of the pharynx. The superior pharyngeal constrictor is largely in the nasopharynx. It has several origins (some texts regard it as more than one muscle) one of which is the medial pterygoid plate. It assists in the constriction of the nasopharynx, but has little role in speech production other than helping form a site against which the velum may be pulled when forming a velic closure. The medial pharyngeal constrictor, which originates on the greater horn of the hyoid bone, also has little function in speech. To some extent it can be considered as an elevator of the hyoid bone, but its most important role for speech is simply as the back wall of the vocal tract. The inferior pharyngeal constrictor also performs this function, but plays a more important role constricting the pharynx in the formation of pharyngeal consonants. -

Head and Neck Specimens
Head and Neck Specimens DEFINITIONS AND GENERAL COMMENTS: All specimens, even of the same type, are unique, and this is particularly true for Head and Neck specimens. Thus, while this outline is meant to provide a guide to grossing the common head and neck specimens at UAB, it is not all inclusive and will not capture every scenario. Thus, careful assessment of each specimen with some modifications of what follows below may be needed on a case by case basis. When in doubt always consult with a PA, Chief/Senior Resident and/or the Head and Neck Pathologist on service. Specimen-derived margin: A margin taken directly from the main specimen-either a shave or radial. Tumor bed margin: A piece of tissue taken from the operative bed after the main specimen has been resected. This entire piece of tissue may represent the margin, or it could also be specifically oriented-check specimen label/requisition for any further orientation. Margin status as determined from specimen-derived margins has been shown to better predict local recurrence as compared to tumor bed margins (Surgical Pathology Clinics. 2017; 10: 1-14). At UAB, both methods are employed. Note to grosser: However, even if a surgeon submits tumor bed margins separately, the grosser must still sample the specimen margins. Figure 1: Shave vs radial (perpendicular) margin: Figure adapted from Surgical Pathology Clinics. 2017; 10: 1-14): Red lines: radial section (perpendicular) of margin Blue line: Shave of margin Comparison of shave and radial margins (Table 1 from Chiosea SI. Intraoperative Margin Assessment in Early Oral Squamous Cell Carcinoma. -
Head and Neck: Summary Stage 2018 Coding Manual V2.0
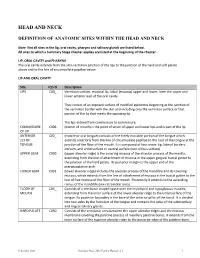
HEAD AND NECK DEFINITION OF ANATOMIC SITES WITHIN THE HEAD AND NECK Note: Not all sites in the lip, oral cavity, pharynx and salivary glands are listed below. All sites to which a Summary Stage chapter applies are listed at the beginning of the chapter. LIP, ORAL CAVITY and PHARYNX The oral cavity extends from the skin-vermilion junction of the lips to the junction of the hard and soft palate above and to the line of circumvallate papillae below. LIP AND ORAL CAVITY Site ICD-O Description LIPS C00_ Vermilion surface, mucosal lip, labial (mucosa) upper and lower, form the upper and lower anterior wall of the oral cavity. They consist of an exposed surface of modified epidermis beginning at the junction of the vermilion border with the skin and including only the vermilion surface or that portion of the lip that meets the opposing lip. The lips extend from commissure to commissure. COMMISSURE C006 (corner of mouth) is the point of union of upper and lower lips and is part of the lip OF LIP ANTERIOR C02_ (mobile or oral tongue) consists of the freely movable portion of the tongue which 2/3 OF extends anteriorly from the line of circumvallate papillae to the root of the tongue at the TONGUE junction of the floor of the mouth. It is composed of four areas: tip, lateral borders, dorsum, and undersurface or ventral surface (non-villous surface). UPPER GUM C030 (upper alveolar ridge) is the covering mucosa of the alveolar process of the maxilla, extending from the line of attachment of mucosa in the upper gingival buccal gutter to the junction of the hard palate. -

Evaluation and Management of the Patient with a Neck Mass
Head & Neck Tumours Part II Content • Tumours of the Ears • Tumours of the Nose • Tumours of the Mouth • Tumours of the Pharynx • Tumours of the Larynx Neoplasms of the Ear and Lateral Skull Base • Lesions of the Pinna and EAC • Lesions of the Middle Ear and Mastoid • Lesions of the Petrous Apex and Clivus • Lesions of the IAC, CPA, and Skull Base Introduction • Generally classified by location, and occasionally by cell-type • Causes of these neoplasms are largely unknown? Neoplasms of the pinna and external auditory canal Cutaneous carcinoma Osteoma and exostosis Squamous cell carcinoma Basal cell carcinoma Miscellaneous neoplasm Merkel cell carcinoma Squamous papilloma Malignant melanoma Pilomatrixoma Myxoma Glandular neoplasm Auricular endochondrial Ceruminous adenoma pseudocyst Ceruminous adenocarcinoma Chondrodermatitis nodularis Pleomorphic adenoma chronica helicis (Winkler disease) Adenoid cystic carcinoma Lesions of the Petrous Apex and Clivus • Adenomatous neoplasm • Langerhans cell histiocytosis Benign middle ear adenoma Eosinophilic granuloma Endolymphatic sac tumor Hand-Schüller-Christian disease Letterer-Siwe disease Chordoma Sarcoma Rhabdomyosarcoma Chondrosarcoma Congenital neoplasm Ewing sarcoma Dermoid Osteogenic sarcoma Teratoma Fibrosarcoma Choristoma Cholesterol granuloma Neoplasms of the internal auditory canal and cerebellopontine angle Schwannoma Vestibular schwannoma Facial nerve schwannoma Trigeminal schwannoma Jugular foramen schwannoma Meningioma Lipoma Metastases Neoplasms of the Pinna and EAC Cutaneous Carcinoma BCC • BCC (20% of ear/TB neoplasms) • Most on pinna • Sun exposure is initiator • Locally infiltrative, rolled border central crusting ulcer • May invade TB if left untreated Cutaneous Carcinoma SCCA • Pinna and EAC are common • Sun, cold, radiation are all factors • Scaly irregular indurated maculopapular lesion, often ulcerated with sero-sang d/c • Can be confused with OE • Other symptoms VII, CHL, SNHL (with invasion of TB) • Met. -

National Head and Neck Cancer Audit
National Head and Neck Cancer Audit Key findings for England and Wales for the audit period October 2007 to November 2008 DAHNO fourth annual report Prepared in partnership with: National Head and Neck Cancer Audit National Head and Neck Cancer Audit Key findings for England and Wales for the audit period October 2007 to November 2008 This fourth report for the National Head and Neck Cancer Audit presents data collected on new registrations from 1 November 2007 to 31 October 2008 and treatment data up to 23 November 2008. The report reflects findings form the analysis of that data, and provides recommendations for improving data quality and completeness. The National Head and Neck Cancer Audit aims to improve both the volume and quality of data submissions, and from this, provide comparative feedback to NHS Provided Trusts, with the ultimate aim of improving patient care. This year the annual report is only available in electronic format, but is accompanied by a brief printed summary report which will be widely disseminated. An in depth more detailed reference report is also available electronically from the website below, configured for those interested in cumulative data, extended analysis and more extensive references. Electronic copies of both these versions of this report can be found at www.ic/nhs.uk/canceraudits. For further information about this report, email: [email protected] or contact: National Clinical Audit Support Programme (NCASP) The Information Centre for health and social care 1 Trevelyan Square Boar Lane Leeds LS1 6AE Copyright © 2008, The Health and Social Care Information Centre, National Head and Neck Cancer Audit. -

A Retrospective Comparison of Tumor Recurrence and Patient Survival After
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1987 A retrospective comparison of tumor recurrence and patient survival after treatment with preoperative radiation therapy and surgery, or surgery and postoperative radiation therapy for squamous cell carcinoma of the pyriform sinus and hypopharynx Jesse George Wardlow Jr. Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Wardlow, Jesse George Jr., "A retrospective comparison of tumor recurrence and patient survival after treatment with preoperative radiation therapy and surgery, or surgery and postoperative radiation therapy for squamous cell carcinoma of the pyriform sinus and hypopharynx" (1987). Yale Medicine Thesis Digital Library. 3284. http://elischolar.library.yale.edu/ymtdl/3284 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. A RETROSPECTIVE COMPARISON OF Permission for photocopying or microfilming of purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work, or otherwise placing -

Laryngeal and Hypopharyngeal Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis
cancer.org | 1.800.227.2345 Laryngeal and Hypopharyngeal Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Finding cancer early often allows for more successful treatment options. Some early cancers may have signs and symptoms that can be noticed, but that's not always the case. ● Can Laryngeal and Hypopharyngeal Cancers Be Found Early? ● Signs and Symptoms of Laryngeal and Hypopharyngeal Cancers ● Tests for Laryngeal and Hypopharyngeal Cancers Stages and Outlook (Prognosis) After a cancer diagnosis, staging provides important information about the extent of cancer in the body and likely response to treatment. ● Laryngeal Cancer Stages ● Hypopharyngeal Cancer Stages ● Survival Rates for Laryngeal and Hypopharyngeal Cancers Questions to Ask About Laryngeal and Hypopharyngeal Cancer Here are some questions you can ask your cancer care team to help you better understand your cancer diagnosis and treatment options. ● Questions to Ask Your Doctor About Laryngeal or Hypopharyngeal Cancer 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Can Laryngeal and Hypopharyngeal Cancers Be Found Early? Screening is testing for cancer or pre-cancer in people who have no symptoms of the disease. Screening tests may find some types of cancer early, when treatment is most likely to be successful. For now, there is no screening test to find laryngeal and hypopharyngeal cancers early. These cancers are often hard to find and diagnose without complex tests. Because these cancers are not common, and the tests need specialized doctors, neither the American Cancer Society nor any other group recommends routine screening for these cancers. Sometimes though, laryngeal and hypopharyngeal cancers can be found early. -

Shifteh Retropharyngeal Danger and Paraspinal Spaces ASHNR 2016
Acknowledgment • Illustrations Courtesy Amirsys, Inc. Retropharyngeal, Danger, and Paraspinal Spaces Keivan Shifteh, M.D. Professor of Clinical Radiology Director of Head & Neck Imaging Program Director, Neuroradiology Fellowship Montefiore Medical Center Albert Einstein College of Medicine Bronx, New York Retropharyngeal, Danger, and Retropharyngeal Space (RPS) Paraspinal Spaces • It is a potential space traversing supra- & infrahyoid neck. • Although diseases affecting these spaces are relatively uncommon, they can result in significant morbidity. • Because of the deep location of these spaces within the neck, lesions arising from these locations are often inaccessible to clinical examination but they are readily demonstrated on CT and MRI. • Therefore, cross-sectional imaging plays an important role in the evaluation of these spaces. Retropharyngeal Space (RPS) Retropharyngeal Space (RPS) • It is seen as a thin line of fat between the pharyngeal • It is bounded anteriorly by the MLDCF (buccopharyngeal constrictor muscles anteriorly and the prevertebral fascia), posteriorly by the DLDCF (prevertebral fascia), and muscles posteriorly. laterally by sagittaly oriented slips of DLDCF (cloison sagittale). Alar fascia (AF) Retropharyngeal Space • Coronally oriented slip of DLDCF (alar fascia) extends from • The anterior compartment is true or proper RPS and the the medial border of the carotid space on either side and posterior compartment is danger space. divides the RPS into 2 compartments: Scali F et al. Annal Otol Rhinol Laryngol. 2015 May 19. Retropharyngeal Space Danger Space (DS) • The true RPS extends from the clivus inferiorly to a variable • The danger space extends further inferiorly into the posterior level between the T1 and T6 vertebrae where the alar fascia mediastinum just above the diaphragm.