Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Neoceratopsian Dinosaur from the Early Cretaceous of Mongolia And
ARTICLE https://doi.org/10.1038/s42003-020-01222-7 OPEN A neoceratopsian dinosaur from the early Cretaceous of Mongolia and the early evolution of ceratopsia ✉ Congyu Yu 1 , Albert Prieto-Marquez2, Tsogtbaatar Chinzorig 3,4, Zorigt Badamkhatan4,5 & Mark Norell1 1234567890():,; Ceratopsia is a diverse dinosaur clade from the Middle Jurassic to Late Cretaceous with early diversification in East Asia. However, the phylogeny of basal ceratopsians remains unclear. Here we report a new basal neoceratopsian dinosaur Beg tse based on a partial skull from Baruunbayan, Ömnögovi aimag, Mongolia. Beg is diagnosed by a unique combination of primitive and derived characters including a primitively deep premaxilla with four pre- maxillary teeth, a trapezoidal antorbital fossa with a poorly delineated anterior margin, very short dentary with an expanded and shallow groove on lateral surface, the derived presence of a robust jugal having a foramen on its anteromedial surface, and five equally spaced tubercles on the lateral ridge of the surangular. This is to our knowledge the earliest known occurrence of basal neoceratopsian in Mongolia, where this group was previously only known from Late Cretaceous strata. Phylogenetic analysis indicates that it is sister to all other neoceratopsian dinosaurs. 1 Division of Vertebrate Paleontology, American Museum of Natural History, New York 10024, USA. 2 Institut Català de Paleontologia Miquel Crusafont, ICTA-ICP, Edifici Z, c/de les Columnes s/n Campus de la Universitat Autònoma de Barcelona, 08193 Cerdanyola del Vallès Sabadell, Barcelona, Spain. 3 Department of Biological Sciences, North Carolina State University, Raleigh, NC 27695, USA. 4 Institute of Paleontology, Mongolian Academy of Sciences, ✉ Ulaanbaatar 15160, Mongolia. -
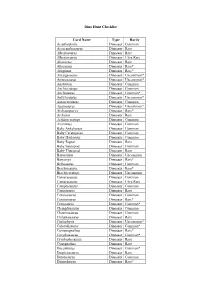
Dino Hunt Checklist Card Name Type Rarity Acanthopholis
Dino Hunt Checklist Card Name Type Rarity Acanthopholis Dinosaur Common Acrocanthosaurus Dinosaur Rare Albertosaurus Dinosaur Rare Albertosaurus Dinosaur Ultra Rare Alioramus Dinosaur Rare Allosaurus Dinosaur Rare* Altispinax Dinosaur Rare* Amargasaurus Dinosaur Uncommon* Ammosaurus Dinosaur Uncommon* Anatotitan Dinosaur Common Anchiceratops Dinosaur Common Anchisaurus Dinosaur Common* Ankylosaurus Dinosaur Uncommon* Antarctosaurus Dinosaur Common Apatosaurus Dinosaur Uncommon* Archaeopteryx Dinosaur Rare* Archelon Dinosaur Rare Arrhinoceratops Dinosaur Common Avimimus Dinosaur Common Baby Ankylosaur Dinosaur Common Baby Ceratopsian Dinosaur Common Baby Hadrosaur Dinosaur Common Baby Raptor Dinosaur Rare Baby Sauropod Dinosaur Common Baby Theropod Dinosaur Rare Barosaurus Dinosaur Uncommon Baryonyx Dinosaur Rare* Bellusaurus Dinosaur Common Brachiosaurus Dinosaur Rare* Brachyceratops Dinosaur Uncommon Camarasaurus Dinosaur Common Camarasaurus Dinosaur Ultra Rare Camptosaurus Dinosaur Common Carnotaurus Dinosaur Rare Centrosaurus Dinosaur Common Ceratosaurus Dinosaur Rare* Cetiosaurus Dinosaur Common* Changdusaurus Dinosaur Common Chasmosaurus Dinosaur Common Chilantaisaurus Dinosaur Rare Coelophysis Dinosaur Uncommon* Coloradisaurus Dinosaur Common* Compsognathus Dinosaur Rare* Corythosaurus Dinosaur Common* Cryolophosaurus Dinosaur Rare Cynognathus Dinosaur Rare Dacentrurus Dinosaur Common* Daspletosaurus Dinosaur Rare Datousaurus Dinosaur Common Deinocheirus Dinosaur Rare* Deinonychus Dinosaur Uncommon* Deinosuchus Dinosaur Rare* Diceratops -

CPY Document
v^ Official Journal of the Biology Unit of the American Topical Association 10 Vol. 40(4) DINOSAURS ON STAMPS by Michael K. Brett-Surman Ph.D. Dinosaurs are the most popular animals of all time, and the most misunderstood. Dinosaurs did not fly in the air and did not live in the oceans, nor on lake bottoms. Not all large "prehistoric monsters" are dinosaurs. The most famous NON-dinosaurs are plesiosaurs, moso- saurs, pelycosaurs, pterodactyls and ichthyosaurs. Any name ending in 'saurus' is not automatically a dinosaur, for' example, Mastodonto- saurus is neither a mastodon nor a dinosaur - it is an amphibian! Dinosaurs are defined by a combination of skeletal features that cannot readily be seen when the animal is fully restored in a flesh reconstruction. Because of the confusion, this compilation is offered as a checklist for the collector. This topical list compiles all the dinosaurs on stamps where the actual bones are pictured or whole restorations are used. It excludes footprints (as used in the Lesotho stamps), cartoons (as in the 1984 issue from Gambia), silhouettes (Ascension Island # 305) and unoffi- cial issues such as the famous Sinclair Dinosaur stamps. The name "Brontosaurus", which appears on many stamps, is used with quotation marks to denote it as a popular name in contrast to its correct scientific name, Apatosaurus. For those interested in a detailed encyclopedic work about all fossils on stamps, the reader is referred to the forthcoming book, 'Paleontology - a Guide to the Postal Materials Depicting Prehistoric Lifeforms' by Fran Adams et. al. The best book currently in print is a book titled 'Dinosaur Stamps of the World' by Baldwin & Halstead. -

And the Origin and Evolution of the Ankylosaur Pelvis
Pelvis of Gargoyleosaurus (Dinosauria: Ankylosauria) and the Origin and Evolution of the Ankylosaur Pelvis Kenneth Carpenter1,2*, Tony DiCroce3, Billy Kinneer3, Robert Simon4 1 Prehistoric Museum, Utah State University – Eastern, Price, Utah, United States of America, 2 Geology Section, University of Colorado Museum, Boulder, Colorado, United States of America, 3 Denver Museum of Nature and Science, Denver, Colorado, United States of America, 4 Dinosaur Safaris Inc., Ashland, Virginia, United States of America Abstract Discovery of a pelvis attributed to the Late Jurassic armor-plated dinosaur Gargoyleosaurus sheds new light on the origin of the peculiar non-vertical, broad, flaring pelvis of ankylosaurs. It further substantiates separation of the two ankylosaurs from the Morrison Formation of the western United States, Gargoyleosaurus and Mymoorapelta. Although horizontally oriented and lacking the medial curve of the preacetabular process seen in Mymoorapelta, the new ilium shows little of the lateral flaring seen in the pelvis of Cretaceous ankylosaurs. Comparison with the basal thyreophoran Scelidosaurus demonstrates that the ilium in ankylosaurs did not develop entirely by lateral rotation as is commonly believed. Rather, the preacetabular process rotated medially and ventrally and the postacetabular process rotated in opposition, i.e., lateral and ventrally. Thus, the dorsal surfaces of the preacetabular and postacetabular processes are not homologous. In contrast, a series of juvenile Stegosaurus ilia show that the postacetabular process rotated dorsally ontogenetically. Thus, the pelvis of the two major types of Thyreophora most likely developed independently. Examination of other ornithischians show that a non-vertical ilium had developed independently in several different lineages, including ceratopsids, pachycephalosaurs, and iguanodonts. -

Two New Stegosaur Specimens from the Upper Jurassic Morrison Formation of Montana, USA
Editors' choice Two new stegosaur specimens from the Upper Jurassic Morrison Formation of Montana, USA D. CARY WOODRUFF, DAVID TREXLER, and SUSANNAH C.R. MAIDMENT Woodruff, D.C., Trexler, D., and Maidment, S.C.R. 2019. Two new stegosaur specimens from the Upper Jurassic Morrison Formation of Montana, USA. Acta Palaeontologica Polonica 64 (3): 461–480. Two partial skeletons from Montana represent the northernmost occurrences of Stegosauria within North America. One of these specimens represents the northernmost dinosaur fossil ever recovered from the Morrison Formation. Consisting of fragmentary cranial and postcranial remains, these specimens are contributing to our knowledge of the record and distribution of dinosaurs within the Morrison Formation from Montana. While the stegosaurs of the Morrison Formation consist of Alcovasaurus, Hesperosaurus, and Stegosaurus, the only positively identified stegosaur from Montana thus far is Hesperosaurus. Unfortunately, neither of these new specimens exhibit diagnostic autapomorphies. Nonetheless, these specimens are important data points due to their geographic significance, and some aspects of their morphologies are striking. In one specimen, the teeth express a high degree of wear usually unobserved within this clade—potentially illuminating the progression of the chewing motion in derived stegosaurs. Other morphologies, though not histologically examined in this analysis, have the potential to be important indicators for maturational inferences. In suite with other specimens from the northern extent of the formation, these specimens contribute to the ongoing discussion that body size may be latitudinally significant for stegosaurs—an intriguing geographical hypothesis which further emphasizes that size is not an undeviating proxy for maturity in dinosaurs. Key words: Dinosauria, Thyreophora, Stegosauria, Jurassic, Morrison Formation, USA, Montana. -

By Howard Zimmerman
by Howard Zimmerman DINO_COVERS.indd 4 4/24/08 11:58:35 AM [Intentionally Left Blank] by Howard Zimmerman Consultant: Luis M. Chiappe, Ph.D. Director of the Dinosaur Institute Natural History Museum of Los Angeles County 1629_ArmoredandDangerous_FNL.ind1 1 4/11/08 11:11:17 AM Credits Title Page, © Luis Rey; TOC, © De Agostini Picture Library/Getty Images; 4-5, © John Bindon; 6, © De Agostini Picture Library/The Natural History Museum, London; 7, © Luis Rey; 8, © Luis Rey; 9, © Adam Stuart Smith; 10T, © Luis Rey; 10B, © Colin Keates/Dorling Kindersly; 11, © Phil Wilson; 12L, Courtesy of the Royal Tyrrell Museum, Drumheller, Alberta; 12R, © De Agostini Picture Library/Getty Images; 13, © Phil Wilson; 14-15, © Phil Wilson; 16-17, © De Agostini Picture Library/The Natural History Museum, London; 18T, © 2007 by Karen Carr and Karen Carr Studio; 18B, © photomandan/istockphoto; 19, © Luis Rey; 20, © De Agostini Picture Library/The Natural History Museum, London; 21, © John Bindon; 23TL, © Phil Wilson; 23TR, © Luis Rey; 23BL, © Vladimir Sazonov/Shutterstock; 23BR, © Luis Rey. Publisher: Kenn Goin Editorial Director: Adam Siegel Creative Director: Spencer Brinker Design: Dawn Beard Creative Cover Illustration: Luis Rey Photo Researcher: Omni-Photo Communications, Inc. Library of Congress Cataloging-in-Publication Data Zimmerman, Howard. Armored and dangerous / by Howard Zimmerman. p. cm. — (Dino times trivia) Includes bibliographical references and index. ISBN-13: 978-1-59716-712-3 (library binding) ISBN-10: 1-59716-712-6 (library binding) 1. Ornithischia—Juvenile literature. 2. Dinosaurs—Juvenile literature. I. Title. QE862.O65Z56 2009 567.915—dc22 2008006171 Copyright © 2009 Bearport Publishing Company, Inc. All rights reserved. -

The Princeton Field Guide to Dinosaurs, Second Edition
MASS ESTIMATES - DINOSAURS ETC (largely based on models) taxon k model femur length* model volume ml x specific gravity = model mass g specimen (modeled 1st):kilograms:femur(or other long bone length)usually in decameters kg = femur(or other long bone)length(usually in decameters)3 x k k = model volume in ml x specific gravity(usually for whole model) then divided/model femur(or other long bone)length3 (in most models femur in decameters is 0.5253 = 0.145) In sauropods the neck is assigned a distinct specific gravity; in dinosaurs with large feathers their mass is added separately; in dinosaurs with flight ablity the mass of the fight muscles is calculated separately as a range of possiblities SAUROPODS k femur trunk neck tail total neck x 0.6 rest x0.9 & legs & head super titanosaur femur:~55000-60000:~25:00 Argentinosaurus ~4 PVPH-1:~55000:~24.00 Futalognkosaurus ~3.5-4 MUCPv-323:~25000:19.80 (note:downsize correction since 2nd edition) Dreadnoughtus ~3.8 “ ~520 ~75 50 ~645 0.45+.513=.558 MPM-PV 1156:~26000:19.10 Giraffatitan 3.45 .525 480 75 25 580 .045+.455=.500 HMN MB.R.2181:31500(neck 2800):~20.90 “XV2”:~45000:~23.50 Brachiosaurus ~4.15 " ~590 ~75 ~25 ~700 " +.554=~.600 FMNH P25107:~35000:20.30 Europasaurus ~3.2 “ ~465 ~39 ~23 ~527 .023+.440=~.463 composite:~760:~6.20 Camarasaurus 4.0 " 542 51 55 648 .041+.537=.578 CMNH 11393:14200(neck 1000):15.25 AMNH 5761:~23000:18.00 juv 3.5 " 486 40 55 581 .024+.487=.511 CMNH 11338:640:5.67 Chuanjiesaurus ~4.1 “ ~550 ~105 ~38 ~693 .063+.530=.593 Lfch 1001:~10700:13.75 2 M. -

A Phylogenetic Analysis of the Basal Ornithischia (Reptilia, Dinosauria)
A PHYLOGENETIC ANALYSIS OF THE BASAL ORNITHISCHIA (REPTILIA, DINOSAURIA) Marc Richard Spencer A Thesis Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements of the degree of MASTER OF SCIENCE December 2007 Committee: Margaret M. Yacobucci, Advisor Don C. Steinker Daniel M. Pavuk © 2007 Marc Richard Spencer All Rights Reserved iii ABSTRACT Margaret M. Yacobucci, Advisor The placement of Lesothosaurus diagnosticus and the Heterodontosauridae within the Ornithischia has been problematic. Historically, Lesothosaurus has been regarded as a basal ornithischian dinosaur, the sister taxon to the Genasauria. Recent phylogenetic analyses, however, have placed Lesothosaurus as a more derived ornithischian within the Genasauria. The Fabrosauridae, of which Lesothosaurus was considered a member, has never been phylogenetically corroborated and has been considered a paraphyletic assemblage. Prior to recent phylogenetic analyses, the problematic Heterodontosauridae was placed within the Ornithopoda as the sister taxon to the Euornithopoda. The heterodontosaurids have also been considered as the basal member of the Cerapoda (Ornithopoda + Marginocephalia), the sister taxon to the Marginocephalia, and as the sister taxon to the Genasauria. To reevaluate the placement of these taxa, along with other basal ornithischians and more derived subclades, a phylogenetic analysis of 19 taxonomic units, including two outgroup taxa, was performed. Analysis of 97 characters and their associated character states culled, modified, and/or rescored from published literature based on published descriptions, produced four most parsimonious trees. Consistency and retention indices were calculated and a bootstrap analysis was performed to determine the relative support for the resultant phylogeny. The Ornithischia was recovered with Pisanosaurus as its basalmost member. -

The Systematic Position of the Enigmatic Thyreophoran Dinosaur Paranthodon Africanus, and the Use of Basal Exemplifiers in Phyl
1 The systematic position of the enigmatic thyreophoran dinosaur Paranthodon africanus, 2 and the use of basal exemplifiers in phylogenetic analysis 3 4 Thomas J. Raven1,2 ,3 and Susannah C. R. Maidment2 ,3 5 61Department of Earth Science & Engineering, Imperial College London, UK 72School of Environment & Technology, University of Brighton, UK 8 3Department of Earth Sciences, Natural History Museum, London, UK 9 10Corresponding author: Thomas J. Raven 11 12Email address: [email protected] 13 14 15 16 17 18 19 20 21ABSTRACT 22 23The first African dinosaur to be discovered, Paranthodon africanus was found in 1845 in the 24Lower Cretaceous of South Africa. Taxonomically assigned to numerous groups since discovery, 25in 1981 it was described as a stegosaur, a group of armoured ornithischian dinosaurs 26characterised by bizarre plates and spines extending from the neck to the tail. This assignment 27that has been subsequently accepted. The type material consists of a premaxilla, maxilla, a nasal, 28and a vertebra, and contains no synapomorphies of Stegosauria. Several features of the maxilla 29and dentition are reminiscent of Ankylosauria, the sister-taxon to Stegosauria, and the premaxilla 30appears superficially similar to that of some ornithopods. The vertebral material has never been 31described, and since the last description of the specimen, there have been numerous discoveries 32of thyreophoran material potentially pertinent to establishing the taxonomic assignment of the 33specimen. An investigation of the taxonomic and systematic position of Paranthodon is therefore 34warranted. This study provides a detailed re-description, including the first description of the 35vertebra. Numerous phylogenetic analyses demonstrate that the systematic position of 36Paranthodon is highly labile and subject to change depending on which exemplifier for the clade 37Stegosauria is used. -

Síntesis Del Registro Fósil De Dinosaurios Tireóforos En Gondwana
ISSN 2469-0228 www.peapaleontologica.org.ar SÍNTESIS DEL REGISTRO FÓSIL DE DINOSAURIOS TIREÓFOROS EN GONDWANA XABIER PEREDA-SUBERBIOLA 1 IGNACIO DÍAZ-MARTÍNEZ 2 LEONARDO SALGADO 2 SILVINA DE VALAIS 2 1Universidad del País Vasco/Euskal Herriko Unibertsitatea, Facultad de Ciencia y Tecnología, Departamento de Estratigrafía y Paleontología, Apartado 644, 48080 Bilbao, España. 2CONICET - Instituto de Investigación en Paleobiología y Geología, Universidad Nacional de Río Negro, Av. General Roca 1242, 8332 General Roca, Río Negro, Ar gentina. Recibido: 21 de Julio 2015 - Aceptado: 26 de Agosto de 2015 Para citar este artículo: Xabier Pereda-Suberbiola, Ignacio Díaz-Martínez, Leonardo Salgado y Silvina De Valais (2015). Síntesis del registro fósil de dinosaurios tireóforos en Gondwana . En: M. Fernández y Y. Herrera (Eds.) Reptiles Extintos - Volumen en Homenaje a Zulma Gasparini . Publicación Electrónica de la Asociación Paleon - tológica Argentina 15(1): 90–107. Link a este artículo: http://dx.doi.org/ 10.5710/PEAPA.21.07.2015.101 DESPLAZARSE HACIA ABAJO PARA ACCEDER AL ARTÍCULO Asociación Paleontológica Argentina Maipú 645 1º piso, C1006ACG, Buenos Aires República Argentina Tel/Fax (54-11) 4326-7563 Web: www.apaleontologica.org.ar Otros artículos en Publicación Electrónica de la APA 15(1): de la Fuente & Sterli Paulina Carabajal Pol & Leardi ESTADO DEL CONOCIMIENTO DE GUIA PARA EL ESTUDIO DE LA DIVERSITY PATTERNS OF LAS TORTUGAS EXTINTAS DEL NEUROANATOMÍA DE DINOSAURIOS NOTOSUCHIA (CROCODYLIFORMES, TERRITORIO ARGENTINO: UNA SAURISCHIA, CON ENFASIS EN MESOEUCROCODYLIA) DURING PERSPECTIVA HISTÓRICA. FORMAS SUDAMERICANAS. THE CRETACEOUS OF GONDWANA. Año 2015 - Volumen 15(1): 90-107 VOLUMEN TEMÁTICO ISSN 2469-0228 SÍNTESIS DEL REGISTRO FÓSIL DE DINOSAURIOS TIREÓFOROS EN GONDWANA XABIER PEREDA-SUBERBIOLA 1, IGNACIO DÍAZ-MARTÍNEZ 2, LEONARDO SALGADO 2 Y SILVINA DE VALAIS 2 1Universidad del País Vasco/Euskal Herriko Unibertsitatea, Facultad de Ciencia y Tecnología, Departamento de Estratigrafía y Paleontología, Apartado 644, 48080 Bilbao, España. -

Ankylosaurid Dinosaur Tail Clubs Evolved Through Stepwise Acquisition of Key Features
1 Title: Ankylosaurid dinosaur tail clubs evolved through stepwise acquisition of key features. 2 3 Victoria M. Arbour1,2,3 and Philip J. Currie3 4 1Paleontology and Geology Research Laboratory, North Carolina Museum of Natural Sciences, Raleigh, 5 North Carolina 27601, USA; 2Department of Biological Sciences, North Carolina State University, Raleigh, 6 North Carolina, 27607, USA; 3Department of Biological Sciences, University of Alberta, Edmonton, 7 Alberta, T6G 2E9, Canada; [email protected] 8 Corresponding author: V. Arbour 9 10 Running title: ANKYLOSAURID TAIL CLUB STEPWISE EVOLUTION 11 12 13 14 15 16 17 18 19 20 21 22 23 24 1 25 ABSTRACT 26 Ankylosaurid ankylosaurs were quadrupedal, herbivorous dinosaurs with abundant dermal 27 ossifications. They are best known for their distinctive tail club composed of stiff, interlocking vertebrae 28 (the handle) and large, bulbous osteoderms (the knob), which may have been used as a weapon. 29 However, tail clubs appear relatively late in the evolution of ankylosaurids, and seemed to have been 30 present only in a derived clade of ankylosaurids during the last 20 million years of the Mesozoic Era. 31 New evidence from mid Cretaceous fossils from China suggests that the evolution of the tail club 32 occurred at least 40 million years earlier, and in a stepwise manner, with early ankylosaurids evolving 33 handle-like vertebrae before the distal osteoderms enlarged and coossified to form a knob. 34 35 Keywords: Dinosauria, Ankylosauria, Ankylosauridae, Cretaceous 36 37 38 39 40 41 42 43 44 45 46 47 48 2 49 INTRODUCTION 50 Tail weaponry, in the form of spikes or clubs, is an uncommon adaptation among tetrapods. -

Dinosaur Species List E to M
Dinosaur Species List E to M E F G • Echinodon becklesii • Fabrosaurus australis • Gallimimus bullatus • Edmarka rex • Frenguellisaurus • Garudimimus brevipes • Edmontonia longiceps ischigualastensis • Gasosaurus constructus • Edmontonia rugosidens • Fulengia youngi • Gasparinisaura • Edmontosaurus annectens • Fulgurotherium australe cincosaltensis • Edmontosaurus regalis • Genusaurus sisteronis • Edmontosaurus • Genyodectes serus saskatchewanensis • Geranosaurus atavus • Einiosaurus procurvicornis • Gigantosaurus africanus • Elaphrosaurus bambergi • Giganotosaurus carolinii • Elaphrosaurus gautieri • Gigantosaurus dixeyi • Elaphrosaurus iguidiensis • Gigantosaurus megalonyx • Elmisaurus elegans • Gigantosaurus robustus • Elmisaurus rarus • Gigantoscelus • Elopteryx nopcsai molengraaffi • Elosaurus parvus • Gilmoreosaurus • Emausaurus ernsti mongoliensis • Embasaurus minax • Giraffotitan altithorax • Enigmosaurus • Gongbusaurus shiyii mongoliensis • Gongbusaurus • Eoceratops canadensis wucaiwanensis • Eoraptor lunensis • Gorgosaurus lancensis • Epachthosaurus sciuttoi • Gorgosaurus lancinator • Epanterias amplexus • Gorgosaurus libratus • Erectopus sauvagei • "Gorgosaurus" novojilovi • Erectopus superbus • Gorgosaurus sternbergi • Erlikosaurus andrewsi • Goyocephale lattimorei • Eucamerotus foxi • Gravitholus albertae • Eucercosaurus • Gresslyosaurus ingens tanyspondylus • Gresslyosaurus robustus • Eucnemesaurus fortis • Gresslyosaurus torgeri • Euhelopus zdanskyi • Gryponyx africanus • Euoplocephalus tutus • Gryponyx taylori • Euronychodon