P R E P R I N T
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dkulturminne I HØYANGER
HISTORISK BAKGRUNN TIL LOKAL KULTURMINNEPLAN dKULTURMINNE I HØYANGER Høyanger kommune eld kraft vatn 2 Historiedel til lokal Kulturminneplan for Høyanger kommune FØREORD Kulturminna våre er viktige kunnskapsberarar som er med på å fortelje oss (pbl), samt utforme områdeplanlegging eller legge tematiske omsynssoner vår historie. Dei kan og vere identitetsberarar som fortel oss kven vi er og der ein ønskjer ei sikra utvikling m.o.t eigenart som arkitektur, bygningsmi - kvar vi kjem frå, og er med på å gi oss identitet og rotfeste. Dei viser utvik - ljø eller sentrumsstruktur. lingstrekk i vår historie, og kan fungere som eit kikhol inn i fortida. Fortidas kulturminne er med oss i notida og gir oss grunnlaget til å skape framtidas Den lokale kulturminneplan gir ikkje noko juridisk vern for kommunal og samfunn. eller privat eigedom. Men ved at Høyanger kommune vedtek den lokale kulturminneplanen vil dette kunne medføre auka midlar gjennom stønads - Kulturminneplanen skal vere eit verktøy i kulturminneforvaltninga. Den skal ordningar og tilgang på rettleiing i bruk og vedlikehald. 3 vere eit hjelpemiddel i ein strategi for å prioritere kulturminne. Ei anna vik - tig side er å kartlegge og formidle kunnskap om våre kulturminne. Dette er Høyanger kommune ønskjer ei god forvaltning av kulturminna i kommu - starten på ein prosess som på sikt vil gje god oversyn og kunnskap om nen. Difor må kulturminna settast inn i ein historisk samanheng. Utarbeida kulturminna i kommunen, både i forvaltninga og formidlinga av kultur - historisk bakgrunn er vedlegg til denne lokale kulturminneplan. minne. God lesing! Kulturminneplanen er i plansystemet til Høyanger kommune, underlagt kommuneplanen sin samfunnsdel, samt arealplanen der ein har bandlagt Arve Varden Anita Nordheim område etter kulturminnelova. -

3-2001 Storsopper I Kommunene Leikanger, Luster Og Sogndal
Storsopper i kommunene Leikanger, Luster og Sogndal registrert under XV Nordiske myko- logiske kongress Sogndal 7-12 september 2000 Tor Erik Brandrud *, Gro Gulden **, Volkmar Timmermann ** & Anders K. Wollan** * Norsk institutt for naturforskning, Postboks 736, 0105 Oslo ** Universitetet i Oslo, NHM - Botanisk museum, Postboks 1172 Blindern, 0318 Oslo Rapport nr. 3 – 2001 ISBN 82-91031-83-5 – ISSN 0803-1886 1 FYLKESMANNEN I SOGN OG FJORDANE Fylkesmannen er Regjeringa og staten sin fremste representant i fylket, og har ansvar for at Stortinget og Regjeringa sine vedtak, mål og retningsliner vert følgde opp. Fylkesman- nen skal fremje fylket sine interesser, ta initiativ både lokalt og overfor sentrale styringsor- gan. Fylkesmannen i Sogn og Fjordane har oppgåver innan landbruk og bygdeutvikling, miljø- vern, på sosialsektoren, overfor kommunane og innan sivil beredskap. Vi er om lag 100 tilsette, og er organisert slik: FYLKESMANN RÅDGJEVAR ADMINISTRA- KOMMKOMMUUNNAALL-- MIMILLJJØØ-- LANDBRUKS- SJONSAVD. AVAVDDEELLININGG VEVERRNN-- AVDELING OG AVAVDDEELLININGG PROSJEKT BERED- VASS- ADMINISTRA- JURIDISK LALANNDD-- SKAP ØKOLOGI SJON ØKOLØKOLOOGGII SSKKOOGGBRBRUKUK JORDBJORDBRURUKK SOSIAL- OG KOMMUNE- OGOG OGOG FAMILIE ØKONOMI ARAREEAALLFFOORRVV.. BYBYGDEGDEUUTTVV.. HER FINN DU OSS: Tinghus III, Skrivarvegen 3, Leikanger Telefon 57 65 50 00 – Telefaks 57 65 50 55 Postadresse: Postboks 37, 6861 Leikanger Landbruksavdelinga: Hafstadgården, Hafstadvegen 48, Førde Telefon: 57 72 32 00 – Telefaks 57 82 12 05 Postadresse: Postboks 14, 6801 Førde E-post: [email protected] Internett: http://www.fjordinfo.no/fmsf/ Framside: Grøn flugesopp, Amanita phalloides. Foto Gro Gulden. 2 Fylkesmannen i Sogn og Fjordane Fylkesmannen i Sogn og Fjorda- ne Rapport nr. 3 – 2001 Forfattar Dato Tor Erik Brandrud *, Gro Gulden **, august 2001 Volkmar Timmermann ** & Anders K. -

Vestland County a County with Hardworking People, a Tradition for Value Creation and a Culture of Cooperation Contents
Vestland County A county with hardworking people, a tradition for value creation and a culture of cooperation Contents Contents 2 Power through cooperation 3 Why Vestland? 4 Our locations 6 Energy production and export 7 Vestland is the country’s leading energy producing county 8 Industrial culture with global competitiveness 9 Long tradition for industry and value creation 10 A county with a global outlook 11 Highly skilled and competent workforce 12 Diversity and cooperation for sustainable development 13 Knowledge communities supporting transition 14 Abundant access to skilled and highly competent labor 15 Leading role in electrification and green transition 16 An attractive region for work and life 17 Fjords, mountains and enthusiasm 18 Power through cooperation Vestland has the sea, fjords, mountains and capable people. • Knowledge of the sea and fishing has provided a foundation Experience from power-intensive industrialisation, metallur- People who have lived with, and off the land and its natural for marine and fish farming industries, which are amongst gical production for global markets, collaboration and major resources for thousands of years. People who set goals, our major export industries. developments within the oil industry are all important when and who never give up until the job is done. People who take planning future sustainable business sectors. We have avai- care of one another and our environment. People who take • The shipbuilding industry, maritime expertise and knowledge lable land, we have hydroelectric power for industry develop- responsibility for their work, improving their knowledge and of the sea and subsea have all been essential for building ment and water, and we have people with knowledge and for value creation. -

Pluscamp Rundreise 2
NordtuNordturr Reise-Service AlternativeReise 1: 2: Westnorwegen und die Stadt Bergen Etappe 1: E39 Bergen - Oppedal Fähre nach Lavik - Vadheim - Sande - Förde - Vassenden, Pluscamp Jölstraholmen. Rundreisen Norwegen Etappe 2: Vassenden - Förde - rv.5 Florö, Pluscamp Krokane. Etappe 3: rv.5 Florö - Storebru - rv. 615 Hyen - Hestenesöyra - Hestenesöyra - Sandane - E39 Byrkelo - rv.60 Innvik - Olden - Loen, Pluscamp Sande. Etappe 4: rv.60 Loen - Stryn - rv.15 Strynefjellet/Grotli - Skåk - Lom - rv. 55 Sognefjellet - Gaupne, Pluscamp Sandvik Etappe 5: rv.55 Gaupne - Sogndal - rv.5 Lærdal- rv.16 Lærdals- tunnellen - Gudvangen - Stalheim - Voss - Bergen, Pluscamp Lone Start in Bergen, Übernachtung im Pluscamp Lone. Lone Camping liegt gleich beim Reichsweg 580, 19 km von Bergen. Mit einer Fläche von 5 ha ist das Bergens grösster Campingplatz. Er liegt idyllisch beim Haukelandsee mit dem Liagebirge (650 m.ü.d.M.) im Hintergrund. 120 Plätze für Campingwagen, Minigolf, Sand- volleyballanlage, Kanuverleih, kleiner Tierpark und Spielplatz. Etappe 1: E39 Bergen - Oppedal Fähre nach Lavik - Vadheim - Sande - Førde - Vassenden Kultur: Håkonshalle · Bryggen · Troldhaugen · Einige Museen und Ausstellungen. Bergen ist Vestlands Für unsere Norwegenrundreisen bevorzugen wir "Hauptstadt", eine Stadtbesichtigung ist dringend anzuraten. Schmale Holzhäuser, Bürger- Plätze von PlusCamp, für welche Sie bei uns auch in steige gehen in steilste Treppenaufgänge über und überall spriesst die Blumenpracht. Bei der Hauptsaison gültige Voucher erhalten können. deiner Bergtour liegt Ihnen Bergen und der Fjord zu Füssen. Machen sie einen Ausflug nach Fløien, zum Aquarium und zur Festung und vergessen sie nicht Fiskebryggen(Fischmarkt), den berühmten Fischmarkt. Auch für einen Besuch von "Troldhaugen", Nina und Edvard Griegs Wohnhaus als auch Lysöen (Ole Bulls Sommerhaus) sollte man sich Zeit nehmen. -

Høyring - Fredingsområde for Hummar I Aurland, Balestrand, Høyanger, Leikanger, Luster, Lærdal, Sogndal Og Vik Kommunar - Sogn Og Fjordane
Aurland kommune Adm.enhet: Forvaltningsseksjonen i region Vest Vangen 1 Sakshandsamar: Hanne Marie Utvær Telefon: 91102779 5745 AURLAND Vår referanse: 18/934 Dykkar referanse: Dato: 26.06.2018 Høyring - fredingsområde for hummar i Aurland, Balestrand, Høyanger, Leikanger, Luster, Lærdal, Sogndal og Vik kommunar - Sogn og Fjordane Fiskeridirektoratet region Vest sender ut forslag til fredingsområder for hummar i Sognefjorden på høyring. Frist for uttale er sett til 31. august 2018. Merknadar til framlegget sender du til [email protected]. Kommunane Høyanger, Vik, Balestrand, Leikanger, Sogndal, Luster, Lærdal og Aurland, har gått saman om forslag til freding av hummar for i alt 17 områder i Sognefjorden. Fredingsområda skal gjelde frå land og ut til 50 meters djup. Innanfor desse områda skal hummaren vere totalfreda. For 14 av områda vil det gjelde forbod mot fiske med fastståande reiskap som teiner, ruser og garn gjennom heile året. For 3 av områda (Sogndalsfjorden, Aurlandsfjorden og Lusterfjorden) vil det gjelde forbod mot hummarteiner gjennom heile året. Fredingsområda vert foreslått innført for 10 år. Om innføring av fredingsområder for hummar Fredingsområder for hummar er eit reguleringstiltak som kjem i tillegg til dei meir tradisjonelle tiltaka som utforming av teiner, grenser på tal teiner og fiskeperiodar. Så langt har innføring av fredingsområder for hummar på Sørlandet vist seg å vere svært positivt for bestandsutviklinga i områda. Ifølge Havforskingsinstituttet (Havforskningsrapporten 2014) har hummarbestanden i løpet av en fireårsperiode økt med 245 prosent, og gjennomsnittstørrelsen har økt med 13 prosent. Å opprette fredingsområder er eit av fleire verkemidlar som bidreg til ein meir berekraftig forvalting av hummar. I 2014 inviterte Fiskeridirektoratet landets kystkommunar med i ein prosess for å etablere nye fredingsområder for hummar. -

Sognefjord in a Nutshell & Unesco Fjord Bus Tour
Sognefjord in a nutshell & Unesco Fjord Bus Tour Please notice! You may also choose other options than the suggested travel routes. We recommend to use 3-5 days on this journey. Travel route, one-way trip from Oslo to Bergen Daily from 24th June to 1st September 2019 Day 1 Train from Oslo 08:25 Oslo Central station, the Bergen Railway To Myrdal 12:58 Change trains Train from Myrdal 13:27 The Flåm Railway To Flåm 14:25 Express boat from Flåm 15:30 Dep. quay no 2 in Flåm To Leikanger 16:25 Change from express boat to bus at Leikanger quay Bus from Leikanger 16:30 Bus has sign for SOGNDAL To Sogndal 16:55 Available time for excursion etc. Overnight stay in Sogndal Day 2 Departure by bus from Sogndal 08:00 (Sogndal bus terminal, located in the centre, 3 min. walk from Quality Hotel Sogndal to the bus terminal) To Fjærland 08:25 Fjærland bus stop (just beside the Glacier Museum To Skei 09:00 Skei bus stop Olden 09:55 Olden bus stop in the centre of Olden Loen 10:15 Loen bus stop (just beside Loen Active) Stryn 10:25 Stryn bus terminal To Geiranger 12:15 Geiranger bus stop (located in the centre, just beside Geiranger fjordservice 12:15 – 15:30 Optional activities kayaking, rib-boat trip, overnight stop Departure by bus from 15:30 Geiranger bus stop, please look for sign with Unesco-bus Geiranger To Stryn 17:15 Stryn bus terminal To Loen 17:30 Loen bus stop (just beside Loen Active) To Olden 17:35 Olden bus stop in the centre of Olden To Skei 18:35 Skei bus stop To Fjærland 19:05 Fjærland bus stop (just beside the Glacier Museum) To Sogndal 19:40 Sogndal bus terminal Overnight stay in Sogndal Day 3 Bus from Sogndal 16:00 Bus has sign for LEIKANGER platform 3 To Leikanger 16:25 Change from bus to express boat Express boat from Leikanger 16:30 To Bergen 20:45 Strandkaiterminalen Bergen (next to the Tourist Information) Travel route, round trip from Oslo via Bergen Day 1 Train from Oslo 08:25 Oslo Central station, the Bergen Railway To Myrdal 12:58 Change trains Train from Myrdal 13:27 The Flåm Railway To Flåm 14:25 Express boat from Flåm 15:30 Dep. -
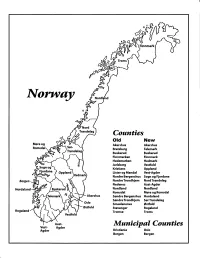
Norway Maps.Pdf
Finnmark lVorwny Trondelag Counties old New Akershus Akershus Bratsberg Telemark Buskerud Buskerud Finnmarken Finnmark Hedemarken Hedmark Jarlsberg Vestfold Kristians Oppland Oppland Lister og Mandal Vest-Agder Nordre Bergenshus Sogn og Fjordane NordreTrondhjem NordTrondelag Nedenes Aust-Agder Nordland Nordland Romsdal Mgre og Romsdal Akershus Sgndre Bergenshus Hordaland SsndreTrondhjem SorTrondelag Oslo Smaalenenes Ostfold Ostfold Stavanger Rogaland Rogaland Tromso Troms Vestfold Aust- Municipal Counties Vest- Agder Agder Kristiania Oslo Bergen Bergen A Feiring ((r Hurdal /\Langset /, \ Alc,ersltus Eidsvoll og Oslo Bjorke \ \\ r- -// Nannestad Heni ,Gi'erdrum Lilliestrom {", {udenes\ ,/\ Aurpkog )Y' ,\ I :' 'lv- '/t:ri \r*r/ t *) I ,I odfltisard l,t Enebakk Nordbv { Frog ) L-[--h il 6- As xrarctaa bak I { ':-\ I Vestby Hvitsten 'ca{a", 'l 4 ,- Holen :\saner Aust-Agder Valle 6rrl-1\ r--- Hylestad l- Austad 7/ Sandes - ,t'r ,'-' aa Gjovdal -.\. '\.-- ! Tovdal ,V-u-/ Vegarshei I *r""i'9^ _t Amli Risor -Ytre ,/ Ssndel Holt vtdestran \ -'ar^/Froland lveland ffi Bergen E- o;l'.t r 'aa*rrra- I t T ]***,,.\ I BYFJORDEN srl ffitt\ --- I 9r Mulen €'r A I t \ t Krohnengen Nordnest Fjellet \ XfC KORSKIRKEN t Nostet "r. I igvono i Leitet I Dokken DOMKIRKEN Dar;sird\ W \ - cyu8npris Lappen LAKSEVAG 'I Uran ,t' \ r-r -,4egry,*T-* \ ilJ]' *.,, Legdene ,rrf\t llruoAs \ o Kirstianborg ,'t? FYLLINGSDALEN {lil};h;h';ltft t)\l/ I t ,a o ff ui Mannasverkl , I t I t /_l-, Fjosanger I ,r-tJ 1r,7" N.fl.nd I r\a ,, , i, I, ,- Buslr,rrud I I N-(f i t\torbo \) l,/ Nes l-t' I J Viker -- l^ -- ---{a - tc')rt"- i Vtre Adal -o-r Uvdal ) Hgnefoss Y':TTS Tryistr-and Sigdal Veggli oJ Rollag ,y Lvnqdal J .--l/Tranbv *\, Frogn6r.tr Flesberg ; \. -

The Jacobson Family from Laerdal Parish, Sogn Og Fjordane County, Norway; Pioneer Norwegian Settlers in Greenwood Township, Vernon County, Wisconsin
THE JACOBSON FAMILY FROM LAERDAL PARISH, SOGN OG FJORDANE COUNTY, NORWAY PIONEER NORWEGIAN SETTLERS IN GREENWOOD TOWNSHIP, VERNON COUNTY, WISCONSIN BY LAWRENCE W. ONSAGER THE LEMONWEIR VALLEY PRESS Mauston, Wisconsin and Berrien Springs, Michigan 2018 COPYRIGHT © 2018 by Lawrence W. Onsager All rights reserved including the right of reproduction in whole or in part in any form, including electronic or mechanical means, information storage and retrieval systems, without permission in writing from the author. Manufactured in the United States of America. -----------------------------------Cataloging in Publication Data----------------------------------- Onsager, Lawrence William, 1944- The Jacobson Family from Laerdal Parish, Sogn og Fjordane County, Norway; Pioneer Norwegian Settlers in Greenwood Township, Vernon County, Wisconsin. Mauston, Wisconsin and Berrien Springs, Michigan: The Lemonweir Valley Press, 2018. 1. Greenwood Township, Vernon County, Wisconsin 2. Jacobson Family 3. Laerdal Township, Sogn og Fjordane County, Norway 4. Greenwood Norwegian-American Settlement in Vernon County, Wisconsin I. Title Tradition claims that the Lemonweir River was named for a dream. Prior to the War of 1812, an Indian runner was dispatched with a war belt of wampum with a request for the Dakotas and Chippewas to meet at the big bend of the Wisconsin River (Portage). While camped on the banks of the Lemonweir, the runner dreamed that he had lost his belt of wampum at his last sleeping place. On waking in the morning, he found his dream to be a reality and he hastened back to retrieve the belt. During the 1820's, the French-Canadian fur traders called the river, La memoire - the memory. The Lemonweir rises in the extensive swamps and marshes in Monroe County. -

Adresseliste Høyringspartar Til Investeringsprogrammet for Fylkesvegenttet Til RTP 2018-2029 Dato: 13
Adresseliste Høyringspartar til investeringsprogrammet for fylkesvegenttet til RTP 2018-2029 Dato: 13. september 2017 Navn Adresse Postnr/poststed E-post Askøy kommune Postboks 323 5323 Kleppestø Askøy Næringslivsforening Storebotn Næringspark, c/o Tabloidsats AS5300 KLEPPESTØ [email protected] Austevoll kommune Kommunehuset 5392 Storebø Austrheim kommune 5943 Austrheim Avinor Bergen lufthavn Postboks 34 5869 Bergen [email protected] Bergen Brannvesen Postboks 7700 5020 Bergen bergen.brannvesen@bergen.kommune.no Bergen bolig- og byplanforening v/ Pelle Engesæter, Postboks 2304 5824 Bergen [email protected] Bergen kommune Postboks 7700 5020 Bergen Bergen Næringsråd Postboks 843 Sentrum 5807 Bergen [email protected] Bergen og Omland friluftsråd Hellebakken 45 5039 Bergen [email protected] Bergen og Omland havnevesen Postboks 6040 5892 Bergen [email protected] Bergensalliansen v/ Richard Taule Postboks 7700 5020 Bergen [email protected] Bergen Reiselivslag Postboks 4055 5835 Bergen [email protected] Bergen Sentrum AS Olav Kyrresgate 11 5014 Bergen [email protected] Bergen Tomteselskap as Solheimsgaten 9 5058 Bergen [email protected] Bergen og Hordaland Turlag Tverrgaten 4 /6 5017 Bergen [email protected] Bjørgvin bispedøme Postboks 1960 5817 Bergen [email protected] Bjørnefjorden Næringsutvikling Postboks 108 5202 Os BKK AS Postboks 7050 5020 Bergen [email protected] Buskerud fylkeskommune, Samferdselsavdelinga Postboks 3563 3007 Drammen [email protected] Bømlo kommune 5430 Bremnes -

Høyanger 5 Walks 4
HØYANGER 5 WALKS 4 Høyanger. Foto Veri Media. 3 1 2 6 © VERI Media 13 7 8 Lavik. Foto Veri Media. 12 9 www.sognefjord.no Maps: www.ut.no 11 10 NACo, and it was the haunt for many single men. Here were buildings such as “Murgården” (“the Brick House”), “Slottet” (“the Castle”) and “Folkekjøkkenet” (“the People’s Kitchen”). Sæbøtangen was a mix of dwellings established by NACo, but also included private dwellings and businesses. This was a distinctive little quarter, and those who grew up in old Sæbøtangen tell of a lively local community. Some remained here all their lives, but for most it was a temporary solution. In the 1970s and 80s, most of old Sæbøtangen was demolished. 10 Parken (“the Park”) The area Parken was developed between 1916–1928 by NACo and was designed by architect Nicolai Beer. At the bottom of Kloumanns Allé is the Director’s House. He had all of his closest workers and the local management living in the surrounding houses. Throughout The Stairs. Foto Veri Media. the street there are wooden houses for workers, where up to four families could live in each house. The area is largely unchanged since then and is listed as a protected site. The Sogn and Fjordane Bustadbyggelag (“building co-operative”) has taken over all the Walking tour of Høyanger dwellings, with the exception of those facing the sea, which are still owned by the plant. Parken is currently a housing co-operative and employees of Hydro have priority in the Starting point Høyanger Town Square allocation of dwellings. -

Administrative and Statistical Areas English Version – SOSI Standard 4.0
Administrative and statistical areas English version – SOSI standard 4.0 Administrative and statistical areas Norwegian Mapping Authority [email protected] Norwegian Mapping Authority June 2009 Page 1 of 191 Administrative and statistical areas English version – SOSI standard 4.0 1 Applications schema ......................................................................................................................7 1.1 Administrative units subclassification ....................................................................................7 1.1 Description ...................................................................................................................... 14 1.1.1 CityDistrict ................................................................................................................ 14 1.1.2 CityDistrictBoundary ................................................................................................ 14 1.1.3 SubArea ................................................................................................................... 14 1.1.4 BasicDistrictUnit ....................................................................................................... 15 1.1.5 SchoolDistrict ........................................................................................................... 16 1.1.6 <<DataType>> SchoolDistrictId ............................................................................... 17 1.1.7 SchoolDistrictBoundary ........................................................................................... -

Sørdalen Naturreservat I Bremanger Kommune – Løyve Til Maskintransport På Tilkomstveg Til Overføringskanal
Vår dato: Vår ref: 17.12.2020 2020/18716 Dykkar dato: Dykkar ref: 09.12.2020 Sogn og Fjordane Energi Saksbehandlar, innvalstelefon Johannes Anonby, 5764 3125 Att. Ragna Flatla Haugland Sørdalen naturreservat i Bremanger kommune – løyve til maskintransport på tilkomstveg til overføringskanal SFE får løyve til køyring av gravemaskin på anleggsveg gjennom Sørdalen naturreservat for tilkomst til utløpskanal utanfor reservatet Vi viser til søknad i e-post 9.12.2020 Frå Sogn og Fjordane Energiverk, som ønskjer å køyre med gravemaskin på anleggsvegen som går innom Sørdalen naturreservat for å kome til overføringskanal utom reservatet. Søknaden med bakgrunn SFE Produksjon driftar og held ved like kraftverket Svelgen 1 i Bremanger, og no trengst det å utbetre mur i kanalen der overføringstunnelen frå Langevatnet kjem ut i Sørdalen. Erosjon har gjort at nokre større steinar på nordsida delvis har rasa ut. For å få utført arbeidet må det beltast inn ei gravemaskin. Det vil skje på allereie opparbeidd tilkomstveg. I underkant av 100 meter av denne vegen ligg inne i reservatet. Kanalen der reparasjonsarbeidet skal gjerast ligg utanfor naturreservatet. Arbeidet vil skje over 1 eller 2 dagar, og SFE vil varsle oppsyn på førehand. SFE ønskjer eit løyve som gjeld frå d.d. til ut juni månad 2021, sidan det er uvisst om det blir aktuelt å utføre jobben før nyttår eller først etter vinteren (vêr- og føreavhengig). Kanalen vart utbetra i 2015 av Statens Vegvesen i samband med utskifting av kulvert under fylkesveg 614, og Fylkesmannen gav då Vegvesenet dispensasjon, dagsett 23.3.2015, til maskintransport på denne gamle anleggsvegen gjennom verneområdet.