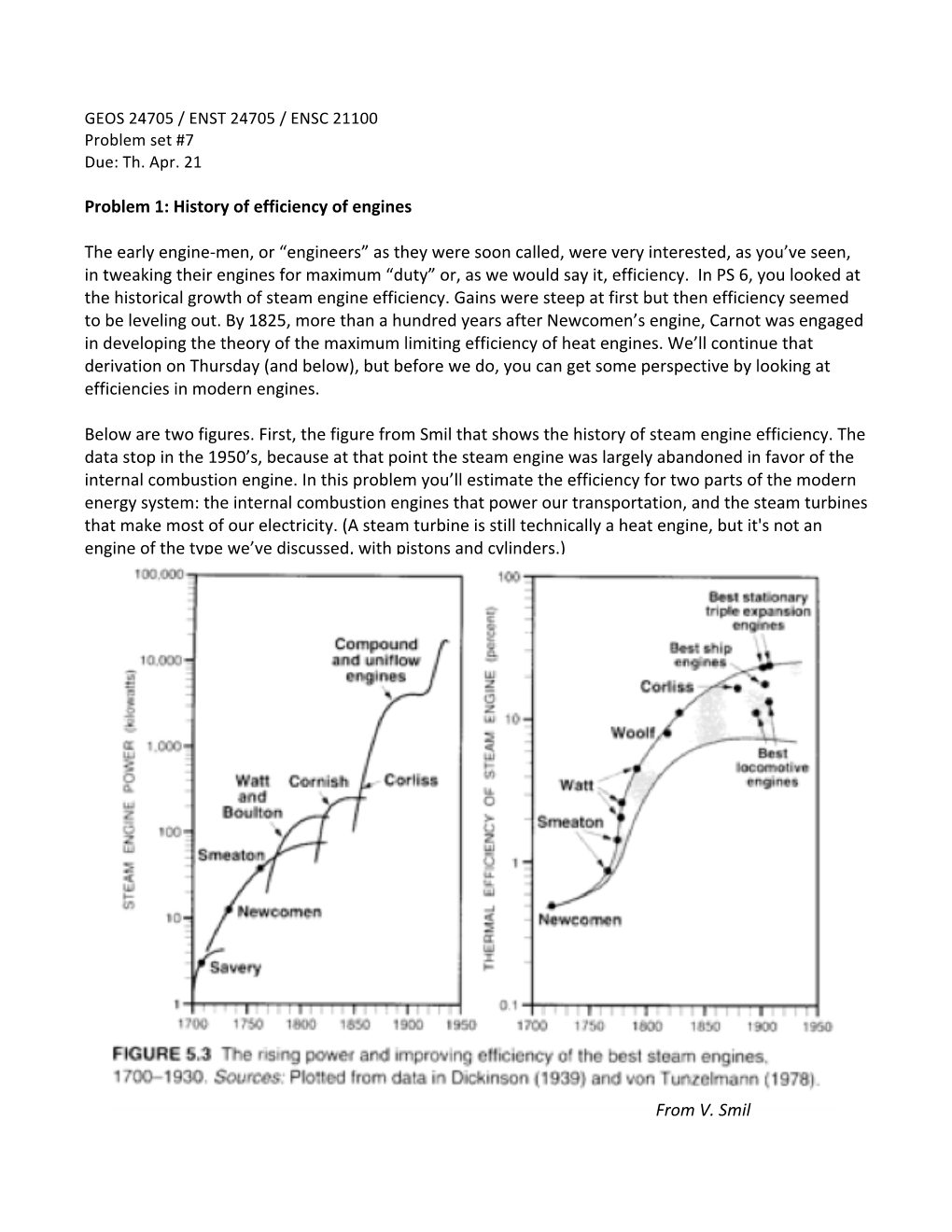
Problem 1: History of Efficiency of Engines the Early Engine-‐Men, Or
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Heavy-Duty Truck Fuel Efficiency Technology Study – Report #2
DOT HS 812 194 February 2016 Commercial Medium- and Heavy-Duty Truck Fuel Efficiency Technology Study – Report #2 This publication is distributed by the U.S. Department of Transportation, National Highway Traffic Safety Administration, in the interest of information exchange. The opinions, findings and conclusions expressed in this publication are those of the author and not necessarily those of the Department of Transportation or the National Highway Traffic Safety Administration. The United States Government assumes no liability for its content or use thereof. If trade or manufacturers’ names or products are mentioned, it is because they are considered essential to the object of the publication and should not be construed as an endorsement. The United States Government does not endorse products or manufacturers. Suggested APA Format Citation: Reinhart, T. E. (2016, February). Commercial medium- and heavy-duty truck fuel efficiency technology study – Report #2. (Report No. DOT HS 812 194). Washington, DC: National Highway Traffic Safety Administration. TECHNICAL REPORT DOCUMENTATION PAGE 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT HS 812 194 4. Title and Subtitle 5. Report Date Commercial Medium- and Heavy-Duty Truck Fuel Efficiency February 2016 Technology Study – Report #2 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Thomas E. Reinhart, Institute Engineer SwRI Project No. 03.17869 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Southwest Research Institute 6220 Culebra Rd. 11. Contract or Grant No. San Antonio, TX 78238 GS-23F-0006M/DTNH22- 12-F-00428 12. Sponsoring Agency Name and Address 13. -

Summary of Current Status of Alternative Fuels and Vehicles
1. INTRODUCTION Motor vehicles are the underlying source for two major global issues the United States (U.S.) faces: 1) the dependence on foreign oil from unstable political regions, and 2) the increase in carbon dioxide (CO2) emissions, a leading contributor of greenhouse gases (GHGs) that affect Earth’s climate. There are 200 million drivers traveling 10 trillion vehicle miles each year in the U.S. In the Denver region alone, vehicles are driven more than 70 million miles each day. Dependence on foreign oil: A matter of U.S. Security The U.S. uses about 14.5 million barrels of oil per day for transportation (which equates to 609 million gallons) and imports more than 60% of its petroleum, two-thirds of which is used to fuel vehicles in the form of gasoline and diesel. The demand for petroleum imports is increasing and with much of the worldwide petroleum resources located in politically volatile countries, the U.S. is vulnerable to supply disruptions. Climate Change While the U.S. contains only 5% of the world’s population, it is responsible for 25% of global GHG emissions. Transportation accounts for 28% of GHG emissions in the U.S., second to electric power Figure 1 (Figure 1). It is the 2006 U.S. Greenhouse Gas Emissions fastest growing by sector (Million Metric Tons CO2 Equivalent) source of GHGs, Residential accounting for 47% of Commericial 5% the net increase in 6% total U.S. GHG Agriculture emissions since 1990. 8% Trends in Colorado are similar, with Electric Power electric power being 34% the lead source of Industry 19% CO2 emissions at 42%, followed by transportation at 31% Transportation (Figure 2). -

High Efficiency VCR Engine with Variable Valve Actuation and New Supercharging Technology
AMR 2015 NETL/DOE Award No. DE-EE0005981 High Efficiency VCR Engine with Variable Valve Actuation and new Supercharging Technology June 12, 2015 Charles Mendler, ENVERA PD/PI David Yee, EATON Program Manager, PI, Supercharging Scott Brownell, EATON PI, Valvetrain This presentation does not contain any proprietary, confidential, or otherwise restricted information. ENVERA LLC Project ID Los Angeles, California ACE092 Tel. 415 381-0560 File 020408 2 Overview Timeline Barriers & Targets Vehicle-Technology Office Multi-Year Program Plan Start date1 April 11, 2013 End date2 December 31, 2017 Relevant Barriers from VT-Office Program Plan: Percent complete • Lack of effective engine controls to improve MPG Time 37% • Consumer appeal (MPG + Performance) Budget 33% Relevant Targets from VT-Office Program Plan: • Part-load brake thermal efficiency of 31% • Over 25% fuel economy improvement – SI Engines • (Future R&D: Enhanced alternative fuel capability) Budget Partners Total funding $ 2,784,127 Eaton Corporation Government $ 2,212,469 Contributing relevant advanced technology Contractor share $ 571,658 R&D as a cost-share partner Expenditure of Government funds Project Lead Year ending 12/31/14 $733,571 ENVERA LLC 1. Kick-off meeting 2. Includes no-cost time extension 3 Relevance Research and Development Focus Areas: Variable Compression Ratio (VCR) Approx. 8.5:1 to 18:1 Variable Valve Actuation (VVA) Atkinson cycle and Supercharging settings Advanced Supercharging High “launch” torque & low “stand-by” losses Systems integration Objectives 40% better mileage than V8 powered van or pickup truck without compromising performance. GMC Sierra 1500 baseline. Relevance to the VT-Office Program Plan: Advanced engine controls are being developed including VCR, VVA and boosting to attain high part-load brake thermal efficiency, and exceed VT-Office Program Plan mileage targets, while concurrently providing power and torque values needed for consumer appeal. -

A Review of Gasoline Compression Ignition
energies Review A Review of Gasoline Compression Ignition: A Promising Technology Potentially Fueled with Mixtures of Gasoline and Biodiesel to Meet Future Engine Efficiency and Emission Targets Yanuandri Putrasari 1,2 and Ocktaeck Lim 3,* 1 Graduate School of Mechanical Engineering, University of Ulsan, San 29, Mugeo2-dong, Nam-gu, Ulsan 44610, Korea; [email protected] or [email protected] 2 Research Centre for Electrical Power and Mechatronics—Indonesian Institute of Sciences, Jl. Cisitu No 154/21D, Bandung 40135, Indonesia 3 School of Mechanical Engineering, University of Ulsan, San 29, Mugeo2-dong, Nam-gu, Ulsan 44610, Korea * Correspondence: [email protected]; Tel.: +82-10-7151-8218 Received: 25 November 2018; Accepted: 5 January 2019; Published: 14 January 2019 Abstract: Efforts have been made to develop efficient and alternative powertrains for internal combustion engines including combustion at low-temperature (LTC) concepts. LTC has been widely studied as a novel combustion mode that offers the possibility to minimize both nitrogen oxide (NOx) and particulate matter (PM) via enhanced air-fuel mixing and intake charge dilution, resulting in lower peak combustion temperatures. Gasoline compression ignition (GCI) is a new ignition method related to the extensive classification of combustion at low-temperature approaches. In this method of ignition, a fuel with high evaporation characteristics and low autoignition sensitivity, for instance gasoline, is burned in a high pressure process. Despite many research efforts, there are still many challenges related with GCI performance for compression ignition (CI) engines. Unstable combustion for idle- to low-load operation was observed because of the low reactivity characteristics of gasoline, and this will affect the efficiency and emissions of the engine. -

Electric Boosting and Energy Recovery Systems for Engine Downsizing
energies Review Electric Boosting and Energy Recovery Systems for Engine Downsizing Mamdouh Alshammari 1,2, Fuhaid Alshammari 2 and Apostolos Pesyridis 1,* 1 Centre of Advanced Powertrain and Fuels (CAPF), Department of Mechanical, Aerospace and Civil Engineering, Brunel University London, Middlesex UB8 3PH, UK; [email protected] 2 Department of Mechanical Engineering, University of Hai’l, Hail 55476, Saudi Arabia; [email protected] * Correspondence: [email protected] Received: 31 October 2019; Accepted: 4 December 2019; Published: 6 December 2019 Abstract: Due to the increasing demand for better fuel economy and increasingly stringent emissions regulations, engine manufacturers have paid attention towards engine downsizing as the most suitable technology to meet these requirements. This study sheds light on the technology currently available or under development that enables engine downsizing in passenger cars. Pros and cons, and any recently published literature of these systems, will be considered. The study clearly shows that no certain boosting method is superior. Selection of the best boosting method depends largely on the application and complexity of the system. Keywords: engine downsizing; electrically assisted turbocharger; electric supercharger; e-turbo; waste heat recovery; turbocharging; supercharging; turbocompounding; organic Rankine cycle 1. Introduction Although internal combustion engines are getting more efficient nowadays, still the major part of fuel energy is transformed into wasted heat. In terms of harmful exhaust emissions, the transportation sector is responsible for the one-third of CO2 emissions worldwide and approximately 15% of the overall greenhouse gas emissions [1]. Moreover, owing to the limited amount of fossil fuels, prices fluctuate significantly, with consistent general rising trends, resulting in economic issues in non-oil-producing countries. -

Designing, Constructing and Testing a Stirling Engine
Designing, Constructing and Testing a Stirling Engine Brock Good advised by Dr. David Heddle Christopher Newport University April 2020 1 Abstract This project was undertaken with the goal of designing a heat engine, which could be built and analyzed, that would utilize the Stirling cycle. The engine was meant to use an input of heat to output work, which could be measured and in turn used to determine specifications about the performance of the en- gine. Some of these specifications include the efficiency, maximum revolutions per minute, and the maximum torque output by the engine. In addition to engine specifications, relationships between the thermodynamic state variables and the measured output quantities of the engine could be observed and an- alyzed. With these goals in mind, over the course of six months, the Stirling engine was designed, modeled, built, tested, and analyzed. The majority of the six month time frame was used for the planning and modelling of potential en- gines, with the final two months spent building the selected design and testing its functionality. 2 Introduction A major reason for the selection of this project was an interest in heat engines, and the utilization of thermodynamic processes to produce work. The Stirling engine specifically, is an example of a very quiet, fairly simple engine, with the prospect for a very high efficiency and a very clean operation. Unlike engines utilizing the Diesel or Otto cycles, such as the petroleum or diesel combustion engines, the Stirling engine dose not require the combustion of any fuel, but rather the transfer of a working fluid. -

Combined Heat and Power: Internal Combustion Engine Technology
Combined Heat and Power INTERNAL COMBUSTION ENGINES Copyright © 2017 Water Environment Federation. All Rights Reserved. WSEC-2017-TR-002, RBC Bioenergy Technology Subcommittee, CHP Task Force 1 Acknowledgments Principal Authors Matthew T. Goss, P.E., CEM, CEA, CDSM, LEED® AP (BD+C), CDM Smith Glynn MacKenzie, P.E. Mechanical Engineer Jason Wiser, P.E. Brown and Caldwell Patrick Wootton, Business Development Manager, Nixon Energy Solutions Myron Bachman, Plant Superintendent, North Davis Sewer District Final Reviewers Kevin R Cowan P.E., District Manager, North Davis Sewer District Tom Phelps, Principal, Stantec David Wrightsman, PE, Business Development Manager, Energy Systems Group Water Environment Federation Residuals and Biosolids Committee, Bioenergy Technology Subcommittee Dru Whitlock, P.E., Vice President, Environmental Engineer, CDM Smith (Subcommittee Chair) Jeffrey Layne, P.E., HRSD (Subcommittee Vice Chair) Copyright © 2017 Water Environment Federation. All Rights Reserved. WSEC-2017-TR-002, RBC Bioenergy Technology Subcommittee, CHP Task Force 2 Table of Contents Acknowledgments .............................................................................................................................................................. 2 Table of Contents ................................................................................................................................................................ 3 Executive Summary ............................................................................................................................................................ -

Improving Economic and Operational Efficiency Through On-Site Application of Advanced Surface Coatings
Industrial gas turbine components Improving economic and operational effi ciency through on-site application of advanced surface coatings anufacturers and operators of power generation equipment M routinely rely on surface coatings to improve engine life and HIÀFLHQF\&ROGVHFWLRQFRDWLQJVSURWHFW airfoil sections against corrosion and wear, limit compressor fouling and help to prevent failure in sensitive and vul- QHUDEOHHQJLQHDUHDV,QWKHKRWVHFWLRQ coatings are used to reduce the effects of hot corrosion and oxidation, and in VRPHFDVHVWRDFWDVDWKHUPDOEDUULHU Failure of unprotected components can trigger a chain reaction within the turbine that could result in catastrophic IDLOXUH Traditionally, components have been shipped to an off-site facility for FRDWLQJ,QWKHWUDGLWLRQDORIIVLWH method, servicing and coating engine components can be a time-consuming SURFHVV&RPSRQHQWVPXVWEHGH stacked, shipped, coated, reshipped DQGUHVWDFNHG'LVDVVHPEOLQJDORQH On-site surface coating of power generation components—in both the cold and hot sections—can shorten outage time, reduce damage risk and increase turbine effi ciency. can involve hundreds of individual SDUWV Large industrial turbine components Table of contents and assemblies such as compressor ,QFUHDVHWXUELQHHIÀFLHQF\ ______________________________ 2 rotors, stators, bladed disks and cases Overview of on-site services _____________________________2 can now be coated on-site, at your ீ &RDWLQJVHUYLFHVWDEOH __________________________________ 3 SRZHUSODQW3UD[DLU·V$[[HVV on-site coating services can help: Fouling -

Alternative Fuel Vehicle Forecasts Final Report
Alternative Fuel Vehicle Forecasts Final report PRC 14-28F Alternative Fuel Vehicle Forecasts Texas A&M Transportation Institute PRC 14-28F April 2016 Authors Richard “Trey” Baker Lauren Cochran Nick Norboge Maarit Moran Jason Wagner Beverly Storey 2 Table of Contents List of Figures ................................................................................................................................ 5 List of Tables ................................................................................................................................. 6 List of Acronyms ........................................................................................................................... 7 Executive Summary ...................................................................................................................... 8 Alternative Fuel Technologies .................................................................................................... 8 Factors Affecting Adoption of Alternative Fuel Vehicles ........................................................ 10 Forecast ..................................................................................................................................... 11 Implications for the State’s Fuel Tax Revenues ........................................................................ 13 Introduction ................................................................................................................................. 15 Texas Funding Implications ...................................................................................................... -

Internal Combustion Engine Supercharging: Turbocharger Vs
Cent. Eur. J. Eng. • 4(2) • 2014 • 110-118 DOI: 10.2478/s13531-013-0133-6 Central European Journal of Engineering Internal combustion engine supercharging: turbocharger vs. pressure wave compressor. Performance comparison Topical Issue: AMMA 2013 Atanasiu Catalin George1∗, Anghel Chiru1 1 ”Transilvania” University of Brasov, Brasov, Romania Received 30 May 2013; accepted 24 September 2013 Abstract: This paper aims on comparison between a turbocharged engine and a pressure wave charged engine. The comparison was accomplished using the engine simulation software AVL Boost, version 2010. The grahps were extracted using AVL Impress, version 2010. The performance increase is limited by the mechanical side of the simulated engine. Keywords: Supercharging• Turbocharger• PWS, Engine• Comparison © Versita sp. z o.o. 1. Introduction The will for the automotive engine builders to manufacture thermic engines with higher and higher efficiencies, but with lower emissions and lower fuel demanding, has led to research intensifications in terms of combustion improvement and geometrical optimization [1]. By comparing the internal combustion engines, in terms of efficiency, we can clearly see that the maximum efficiency has the diesel engine, or the compression-ignition engine. The spark ignition engine has an overall efficiency slightly Figure 1. Thermic engine efficiency comparison. lower. It is followed by the Stirling engine, gas turbine and gas machine (Figure 1). Although the electric engine has an efficiency of 85%, this does not compensate the losses of the electrical energy build-up [2, 3]. including transport, is 29.3%. The same calculation made for coal has an overall efficiency of 31.1%. In this case, The efficiency to convert the oil into electrical energy, the electric engine powered by oil-source energy is 24.9%, and powered by coal-source energy is 26.4% [2]. -

Co-Optimization of Fuels & Engines
Co-Optimization of Fuels & Engines FOR TOMORROW’S ENERGY-EFFICIENT VEHICLES CO-OPTIMIZATION FOR NEAR- AND The initiative’s integrated approach combines the previously indepen- LONG-TERM TRANSPORTATION SOLUTIONS dent areas of biofuels and combustion R&D, bringing together two DOE A new U.S. Department of Energy (DOE) initiative is accelerating the Office of Energy Efficiency & Renewable Energy research offices, nine introduction of affordable, scalable, and sustainable biofuels and national laboratories, and numerous industry and academic partners high-efficiency, low-emission vehicle engines. The simultaneous fuels to more rapidly identify commercially viable solutions. This multi-year and vehicles research and development (R&D) is designed to deliver project will provide industry with the scientific underpinnings required maximum energy savings, emissions reduction, and on-road vehicle to move new biofuels and advanced engine systems to market faster performance. Although improvements over the years based on either while identifying and addressing barriers to their commercialization. fuel or engine advancements have made vehicles cleaner and more fuel efficient, transportation still accounts for 70% of overall U.S. petroleum This coordinated R&D effort complements established initiatives and consumption and 27% of the country’s greenhouse gas (GHG) emissions. partnerships. In addition to exploring synergies among the fuels, en- This project’s ambitious, first-of-its-kind approach simultaneously tackles gines, and powertrains found in today’s traditional light-duty ICE vehi- fuel and engine innovation to co-optimize performance of both elements cles, the initiative is also examining opportunities related to hybrid and and provide dramatic and rapid cuts in fuel use and emissions. -

Airplane Flying Handbook (FAA-H-8083-3B) Chapter 15
Chapter 15 Transition to Jet-Powered Airplanes Introduction This chapter contains an overview of jet powered airplane operations. The information contained in this chapter is meant to be a useful preparation for, and a supplement to, formal and structured jet airplane qualification training. The intent of this chapter is to provide information on the major differences a pilot will encounter when transitioning to jet powered airplanes. In order to achieve this in a logical manner, the major differences between jet powered airplanes and piston powered airplanes have been approached by addressing two distinct areas: differences in technology, or how the airplane itself differs; and differences in pilot technique, or how the pilot addresses the technological differences through the application of different techniques. For airplane-specific information, a pilot should refer to the FAA-approved Airplane Flight Manual for that airplane. 15-1 Jet Engine Basics Although the propeller-driven airplane is not nearly as efficient as the jet, particularly at the higher altitudes and cruising A jet engine is a gas turbine engine. A jet engine develops speeds required in modern aviation, one of the few advantages thrust by accelerating a relatively small mass of air to very the propeller-driven airplane has over the jet is that maximum high velocity, as opposed to a propeller, which develops thrust is available almost at the start of the takeoff roll. Initial thrust by accelerating a much larger mass of air to a much thrust output of the jet engine on takeoff is relatively lower slower velocity. and does not reach peak efficiency until the higher speeds.