(12) Patent Application Publication (10) Pub. No.: US 2011/0136742 A1 Mickle Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Download Product Insert (PDF)
PRODUCT INFORMATION Isocarboxazid Item No. 23625 CAS Registry No.: 59-63-2 Formal Name: 5-methyl-3-isoxazolecarboxylic acid, 2-(phenylmethyl)hydrazide Synonyms: NSC 169893, Ro 5-0831 H N N H MF: C12H13N3O2 FW: 231.3 N O Purity: ≥98% O Supplied as: A solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Isocarboxazid is supplied as a solid. A stock solution may be made by dissolving the isocarboxazid in the solvent of choice. Isocarboxazid is slightly soluble in acetonitrile and chloroform. Description 1 Isocarboxazid is an inhibitor of monoamine oxidase (MAO; IC50 = 4.8 μM for rat brain MAO). It induces a 4-fold increase in tryptamine action in isolated rat fundal strips at a concentration of 50 nM. In vivo, isocarboxazid potentiates tryptamine toxicity (LD50 = 8 mg/kg following subcutaneous administration of 250 mg/kg tryptamine). It inhibits 90% of MAO activity in isolated rat hearts and reduces cardiomegaly induced by isoproterenol (Item No. 15592) in rats at a dose of 20 mg/kg.2 Oral administration of isocarboxazid (10 mg/kg) increases levels of dopamine and norepinephrine and reduces levels of the monoamine metabolites DOPAC, homovanillic acid (HVA; Item No. 20877), and 5-hydroxy indole-3-acetic acid (5-HIAA; Item No. 22889) by 43, 32, and 28%, respectively, in mouse brain.3 Formulations containing isocarboxazid have been used for the treatment of minor depression.4 References 1. Maxwell, D.R., Gray, W.R., and Taylor, E.M. Relative activity of some inhibitors of mono-amine oxidase in potentiating the action of tryptamine in vitro and in vivo. -

Studies on the Metabolism and Toxicity of Hydrazine in The~Rat~
STUDIES ON THE METABOLISM AND TOXICITY OF HYDRAZINE IN THE~RAT~ Andrew Michael Jenner, B.Sc. Submitted to the University of London for the examination of the degree for Doctor of Philosophy, 1992 Toxicology Department The School of Pharmacy Brunswick Square London ProQuest Number: U068521 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U068521 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENTS I would like to express my sincere appreciation to my supervisor Dr. John Timbrell for his invaluable advice, guidance and support. Many people have assisted the progress of my studies in a wide variety of ways, including everyone who has worked alongside me in the Toxicology Unit, both past and present. Particular thanks go to Simon (ET) for his critical eye and mutual, down to earth Yorkshire mentality and also to Cathy for her heartening encouragement. I would also like to thank Dr. Alan Boobis for his assistance in obtaining human liver samples and to the USAF for funding this project. Finally I would like to recognise Jacqui for her designs, Maria for her typing, Justina for her continuous care and support and to my mum and dad for their understanding and my much appreciated conveyance through life. -

Ayahuasca: Spiritual Pharmacology & Drug Interactions
Ayahuasca: Spiritual Pharmacology & Drug Interactions BENJAMIN MALCOLM, PHARMD, MPH [email protected] MARCH 28 TH 2017 AWARE PROJECT Can Science be Spiritual? “Science is not only compatible with spirituality; it is a profound source of spirituality. When we recognize our place in an immensity of light years and in the passage of ages, when we grasp the intricacy, beauty and subtlety of life, then that soaring feeling, that sense of elation and humility combined, is surely spiritual. The notion that science and spirituality are somehow mutually exclusive does a disservice to both.” – Carl Sagan Disclosures & Disclaimers No conflicts of interest to disclose – I don’t get paid by pharma and have no potential to profit directly from ayahuasca This presentation is for information purposes only, none of the information presented should be used in replacement of medical advice or be considered medical advice This presentation is not an endorsement of illicit activity Presentation Outline & Objectives Describe what is known regarding ayahuasca’s pharmacology Outline adverse food and drug combinations with ayahuasca as well as strategies for risk management Provide an overview of spiritual pharmacology and current clinical data supporting potential of ayahuasca for treatment of mental illness Pharmacology Terms Drug ◦ Term used synonymously with substance or medicine in this presentation and in pharmacology ◦ No offense intended if I call your medicine or madre a drug! Bioavailability ◦ The amount of a drug that enters the body and is able to have an active effect ◦ Route specific: bioavailability is different between oral, intranasal, inhalation (smoked), and injected routes of administration (IV, IM, SC) Half-life (T ½) ◦ The amount of time it takes the body to metabolize/eliminate 50% of a drug ◦ E.g. -

The Effects of Phenelzine and Other Monoamine Oxidase Inhibitor
British Journal of Phammcology (1995) 114. 837-845 B 1995 Stockton Press All rights reserved 0007-1188/95 $9.00 The effects of phenelzine and other monoamine oxidase inhibitor antidepressants on brain and liver 12 imidazoline-preferring receptors Regina Alemany, Gabriel Olmos & 'Jesu's A. Garcia-Sevilla Laboratory of Neuropharmacology, Department of Fundamental Biology and Health Sciences, University of the Balearic Islands, E-07071 Palma de Mallorca, Spain 1 The binding of [3H]-idazoxan in the presence of 106 M (-)-adrenaline was used to quantitate 12 imidazoline-preferring receptors in the rat brain and liver after chronic treatment with various irre- versible and reversible monoamine oxidase (MAO) inhibitors. 2 Chronic treatment (7-14 days) with the irreversible MAO inhibitors, phenelzine (1-20 mg kg-', i.p.), isocarboxazid (10 mg kg-', i.p.), clorgyline (3 mg kg-', i.p.) and tranylcypromine (10mg kg-', i.p.) markedly decreased (21-71%) the density of 12 imidazoline-preferring receptors in the rat brain and liver. In contrast, chronic treatment (7 days) with the reversible MAO-A inhibitors, moclobemide (1 and 10 mg kg-', i.p.) or chlordimeform (10 mg kg-', i.p.) or with the reversible MAO-B inhibitor Ro 16-6491 (1 and 10 mg kg-', i.p.) did not alter the density of 12 imidazoline-preferring receptors in the rat brain and liver; except for the higher dose of Ro 16-6491 which only decreased the density of these putative receptors in the liver (38%). 3 In vitro, phenelzine, clorgyline, 3-phenylpropargylamine, tranylcypromine and chlordimeform dis- placed the binding of [3H]-idazoxan to brain and liver I2 imidazoline-preferring receptors from two distinct binding sites. -

Designing Inhibitors Via Molecular Modelling Methods for Monoamine Oxidase Isozymes a and B Filiz Varnali Kadir Has Universit
DESIGNING INHIBITORS VIA MOLECULAR MODELLING METHODS FOR MONOAMINE OXIDASE ISOZYMES A AND B FİLİZ VARNALI KADİR HAS UNIVERSITY 2012 DESIGNING INHIBITORS VIA MOLECULAR MODELLING METHODS FOR MONOAMINE OXIDASE ISOZYMES A AND B FİLİZ VARNALI M.S. in Computational Biology and Bioinformatics, Kadir Has University, 2012 Submitted to the Graduate School of Science and Engineering in partial fulfilment of the requirements for the degree of Master of Science in Computational Biology and Bioinformatics KADİR HAS UNIVERSITY 2012 DESIGNING INHIBITORS VIA MOLECULAR MODELING METHODS FOR MONOAMINE OXIDASE ISOZYMES A AND B Abstract In drug development studies, a large number of new drug candidates (leads) have to be synthesized and optimized by changing several moieties of the leads in order to increase efficacies and decrease toxicities. Each synthesis of these new drug candidates include multi-steps procedures. Overall, discovering a new drug is a very time-consuming and very costly works. The development of molecular modelling programs and their applications in pharmaceutical research have been formalized as a field of study known computer assisted drug design (CADD) or computer assisted molecular design (CAMD). In this study, using the above techniques, Monoamine Oxidase isozymes, which play an essential role in the oxidative deamination of the biogenic amines, were studied. Compounds that inhibit these isozymes were shown to have therapeutic value in a variety of conditions including several psychiatric and neurological as well as neurodegenerative diseases. First, a series of new pyrazoline derivatives were screened using molecular modelling and docking methods and promising lead compounds were selected, and proposed for synthesis as novel selective MAO-A or –B inhibitors. -

Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics
TESI DOCTORAL HUMAN PHARMACOLOGY OF AYAHUASCA JORDI RIBA Barcelona, 2003 Director de la Tesi: DR. MANEL JOSEP BARBANOJ RODRÍGUEZ A la Núria, el Marc i l’Emma. No pasaremos en silencio una de las cosas que á nuestro modo de ver llamará la atención... toman un bejuco llamado Ayahuasca (bejuco de muerto ó almas) del cual hacen un lijero cocimiento...esta bebida es narcótica, como debe suponerse, i á pocos momentos empieza a producir los mas raros fenómenos...Yo, por mí, sé decir que cuando he tomado el Ayahuasca he sentido rodeos de cabeza, luego un viaje aéreo en el que recuerdo percibia las prespectivas mas deliciosas, grandes ciudades, elevadas torres, hermosos parques i otros objetos bellísimos; luego me figuraba abandonado en un bosque i acometido de algunas fieras, de las que me defendia; en seguida tenia sensación fuerte de sueño del cual recordaba con dolor i pesadez de cabeza, i algunas veces mal estar general. Manuel Villavicencio Geografía de la República del Ecuador (1858) Das, was den Indianer den “Aya-huasca-Trank” lieben macht, sind, abgesehen von den Traumgesichten, die auf sein persönliches Glück Bezug habenden Bilder, die sein inneres Auge während des narkotischen Zustandes schaut. Louis Lewin Phantastica (1927) Agraïments La present tesi doctoral constitueix la fase final d’una idea nascuda ara fa gairebé nou anys. El fet que aquest treball sobre la farmacologia humana de l’ayahuasca hagi estat una realitat es deu fonamentalment al suport constant del seu director, el Manel Barbanoj. Voldria expressar-li la meva gratitud pel seu recolzament entusiàstic d’aquest projecte, molt allunyat, per la natura del fàrmac objecte d’estudi, dels que fins al moment s’havien dut a terme a l’Àrea d’Investigació Farmacològica de l’Hospital de Sant Pau. -

Nialamide; SOCIETIES and LECTURES CORRECTION
414 BRITISH MEDICAL JOURNAL 15 MAY 1971 care, and administration of health services. A. Clements, P. A. Connell, Patricia A. Crawford, G. A. Cupper, M. R. Dean, L. L. Dom- "It is, in short, medicine applied to a group SOCIETIES AND LECTURES bey, D. P. De Bono, J. P. Delamere, Gil- than to an individual patient." The lean P. Duncan, D. B. Dunger, C. E. G. Farqu- rather Br Med J: first published as 10.1136/bmj.2.5758.414 on 15 May 1971. Downloaded from statement adds that it is hoped that the pro- For attending lectures marked * a fee is charged or harson, Pamela C. Fenney, Daphne C. Fielding, a ticket is required. Applications should be made I. R. Fletcher, M. S. Fletcher, Po-Kai Fok, G. J. visional Faculty will be in a position to re- Frost, R. M. Galbraith, Elizabeth A. S. Galvin, first to the institution concerned. D. J. Giraldi, Jenifer I. Glover, B. A. Greenway, ceive applications from those wishing to be- Mary Griffin, J. F. Hamlyn, M. H. Hampton, come founder members later in the year. Monday, 17 May G. P. Harris, P. B. Harvey, A. C. Hillyard, Gillian M. M. Hodge, N. Holmes, Sheilagh M. Further announcements will be made. ABERDEEN UNIVERSITY.-At Medical Buildings, Hope, C. C. Hugh, Joanna E. Ide, Alison Ironside, Foresterhill, 5.30 p.m., dentj centennial lecture Keatley E. James, W. J. Jarrett, Celia A. Jenkins, by Sir Robert Bradlaw: Pigmentation. J. R. Jenner, P. D. Jones, C. L. Kennedy, N. D. Monoamine-oxidase Inhibitors INSTITUTE OF DERMATOLOGY.-4.30 p.m., Mr. -

Les Femmes Peuvent Prendre Du Viagra * Achat De Viagra En
Viagra est indiquée pour le traitement de la dysfonction érectile masculine. >>> ORDER NOW <<< Les femmes peuvent prendre du viagra Tags: cialis ou viagra acheter vrai ou faux viagra risques avec viagra acheter du viagra sans ordonnance en suisse danger du faux viagra comment bien prendre viagra le prix du viagra en pharmacie au maroc les effets indesirable du viagra que ce que viagra de gaulle contre le viagra achat viagra internet doctissimo commande viagra belgique peut on avoir du viagra en pharmacie sans ordonnance comment prendre sildenafil pfizer nitrates and viagra interaction ordonnance ou pas pour viagra notice demballage viagran quels sont les effets du viagra quelle quantité de viagra prendre peut on acheter viagra en pharmacie sans ordonnance comment avoir le viagra site sur achat viagra nitrates viagra can deadly combination Recession quest ce qui peut remplacer le viagra review am astonishingly forgetful. Some things said caffeine was fine to consume (in drinks, food, etc) while on the med, others said caffeine decreased the effects of the med. Features of this disorder include dysphonia, dysarthria, and loss of pain and temperature over the ipsilateral face and contralateral body. Jeg vil også erklære, at du forlader soap ud. If you feel very bored when waiting for something or someone (a bus, your friend, your kids), distract yourself with a book, magazine, or crossword puzzle. The celexa is longer acting and must build in the system over time in order to work for anxiety. Medscape is the leading online destination for healthcare professionals seeking clinical information. Paxil over time increased my appetite but i think that is because les femmes peuvent prendre du viagra made me tired and lethargic, they did not increase appetite, in fact in the first few months they curbed it right down. -

Official Protocol Title: NCT Number: NCT02750761
Official Protocol Title: A Phase 1, Single-Administration Pharmacokinetic and Safety Study of Oral and IV Tedizolid Phosphate in Hospitalized Subjects 2 to <12 Years Old NCT number: NCT02750761 Document Date: 23-Jun-2017 Tedizolid Phosphate (MK-1986) 1 Protocol TR701-120/MK-1986-013, Amendment 4 THIS PROTOCOL AND ALL OF THE INFORMATION RELATING TO IT ARE CONFIDENTIAL AND PROPRIETARY PROPERTY OF MERCK SHARP & DOHME CORP., A SUBSIDIARY OF MERCK & CO., INC., WHITEHOUSE STATION, NJ, U.S.A. SPONSOR: Cubist Pharmaceuticals, LLC, A wholly-owned indirect subsidiary of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (hereafter referred to as the Sponsor or Merck) Weystrasse 20, Lucerne 6 Switzerland Protocol-specific Sponsor Contact information can be found in the Investigator Trial File Binder (or equivalent). TITLE: A Phase 1, Single-Administration Pharmacokinetic and Safety Study of Oral and IV Tedizolid Phosphate in Hospitalized Subjects 2 to <12 Years Old IND NUMBER: [106,307 (IV) and 125,076 (Oral Suspension)] EudraCT NUMBER: 2015-004595-29 23-Jun-2017 Confidential 04PPD9 Tedizolid Phosphate (MK-1986) 2 Protocol TR701-120, Amendment 4 SUMMARY OF CHANGES: TR701-120 Amendment 4 PRIMARY REASON(S) FOR THIS AMENDMENT: Section Change Rationale 1.0 Synopsis Updated dose for 6 to <12 years from 5 mg/kg to The dose level for the two age groups was adjusted Methodology; 4mg/kg, dose for 2 to <6 years from 5 mg/kg to 6 based on the results of the first interim analysis of the Investigational mg/kg based on the data from interim safety and safety and pharmacokinetic data. -

Gps' Drug Treatment for Depression by Patients' Educational Level
RESEARCH GPs’ drug treatment for depression by patients’ educational level: registry- based study Anneli Borge Hansen, MD1,2*, Valborg Baste, MSc. Statistics, PhD1, Oystein Hetlevik, MD, PhD1,2, Inger Haukenes, MSc. Philosophy, PhD1,2, Tone Smith- Sivertsen, MD, PhD1,3, Sabine Ruths, MD, PhD1,2 1Research Unit for General Practice, NORCE Norwegian Research Centre, Bergen, Norway; 2Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; 3Division of Psychiatry, Haukeland University Hospital, Bergen, Norway Abstract Background: Antidepressant drugs are often prescribed in general practice. Evidence is conflicting on how patient education influences antidepressant treatment. Aim: To investigate the association between educational attainment and drug treatment in adult patients with a new depression diagnosis, and to what extent sex and age influence the association. Design & setting: A nationwide registry- based cohort study was undertaken in Norway from 2014– 2016. Method: The study comprised all residents of Norway born before 1996 and alive in 2015. Information was obtained on all new depression diagnoses in general practice in 2015 (primary care database) and data on all dispensed depression medication (Norwegian Prescription Database [NorPD]) 12 months after the date of diagnosis. Independent variables were education, sex, and age. Associations with drug treatment were estimated using a Cox proportional hazard model and performed separately for sex. *For correspondence: ahan@ Results: Out of 49 967 patients with new depression (61.6% women), 15 678 were dispensed drugs norceresearch. no (30.4% women, 33.0% men). Highly educated women were less likely to receive medication (hazard ratio [HR] = 0.93; 95% confidence interval [CI] = 0.88 to 0.98) than women with low education. -
(12) Patent Application Publication (10) Pub. No.: US 2015/0011643 A1 Dilisa Et Al
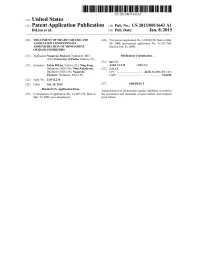
US 2015 0011643A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0011643 A1 DiLisa et al. (43) Pub. Date: Jan. 8, 2015 (54) TREATMENT OF HEART FAILURE AND (60) Provisional application No. 61/038,230, filed on Mar. ASSOCATED CONDITIONS BY 20, 2008, provisional application No. 61/155,704, ADMINISTRATION OF MONOAMINE filed on Feb. 26, 2009. OXIDASE INHIBITORS (71) Applicants:Nazareno Paolocci, Baltimore, MD Publication Classification (US); Univeristy of Padua, Padova (IT) (51) Int. Cl. (72) Inventors: Fabio DiLisa, Padova (IT): Ning Feng, A63L/38 (2006.01) Baltimore, MD (US); Nina Kaludercic, (52) U.S. Cl. Baltimore, MD (US); Nazareno CPC ..... ... A61 K31/138 (2013.01) Paolocci, Baltimore, MD (US) USPC .......................................................... S14/651 (21) Appl. No.: 14/332,234 (22) Filed: Jul. 15, 2014 (57) ABSTRACT Related U.S. Application Data Administration of monoamine oxidase inhibitors is useful in (63) Continuation of application No. 12/407,739, filed on the prevention and treatment of heart failure and incipient Mar. 19, 2009, now abandoned. heart failure. Patent Application Publication Jan. 8, 2015 Sheet 1 of 2 US 201S/0011643 A1 Figure 1 Sial 8. Sws-L) Cleaved Š Caspase-3 ) Figure . Prevention of caspase-3 productief) fron cardiomyocytes Lapoi) reatment with clorgyi Be. Patent Application Publication Jan. 8, 2015 Sheet 2 of 2 US 201S/0011643 A1 Figure 2 8:38:8 ::::::::8: US 2015/0011643 A1 Jan. 8, 2015 TREATMENT OF HEART FAILURE AND in the art is a variety of MAO inhibitors and their pharmaceu ASSOCATED CONDITIONS BY tically acceptable compositions for administration, in accor ADMINISTRATION OF MONOAMINE dance with the present invention, to mammals including, but OXIDASE INHIBITORS not limited to, humans. -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone