Conserved Region I of Human Coactivator TAF4 Binds to a Short Hydrophobic Motif Present in Transcriptional Regulators
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

1 Supporting Information for a Microrna Network Regulates
Supporting Information for A microRNA Network Regulates Expression and Biosynthesis of CFTR and CFTR-ΔF508 Shyam Ramachandrana,b, Philip H. Karpc, Peng Jiangc, Lynda S. Ostedgaardc, Amy E. Walza, John T. Fishere, Shaf Keshavjeeh, Kim A. Lennoxi, Ashley M. Jacobii, Scott D. Rosei, Mark A. Behlkei, Michael J. Welshb,c,d,g, Yi Xingb,c,f, Paul B. McCray Jr.a,b,c Author Affiliations: Department of Pediatricsa, Interdisciplinary Program in Geneticsb, Departments of Internal Medicinec, Molecular Physiology and Biophysicsd, Anatomy and Cell Biologye, Biomedical Engineeringf, Howard Hughes Medical Instituteg, Carver College of Medicine, University of Iowa, Iowa City, IA-52242 Division of Thoracic Surgeryh, Toronto General Hospital, University Health Network, University of Toronto, Toronto, Canada-M5G 2C4 Integrated DNA Technologiesi, Coralville, IA-52241 To whom correspondence should be addressed: Email: [email protected] (M.J.W.); yi- [email protected] (Y.X.); Email: [email protected] (P.B.M.) This PDF file includes: Materials and Methods References Fig. S1. miR-138 regulates SIN3A in a dose-dependent and site-specific manner. Fig. S2. miR-138 regulates endogenous SIN3A protein expression. Fig. S3. miR-138 regulates endogenous CFTR protein expression in Calu-3 cells. Fig. S4. miR-138 regulates endogenous CFTR protein expression in primary human airway epithelia. Fig. S5. miR-138 regulates CFTR expression in HeLa cells. Fig. S6. miR-138 regulates CFTR expression in HEK293T cells. Fig. S7. HeLa cells exhibit CFTR channel activity. Fig. S8. miR-138 improves CFTR processing. Fig. S9. miR-138 improves CFTR-ΔF508 processing. Fig. S10. SIN3A inhibition yields partial rescue of Cl- transport in CF epithelia. -

Molecular Structure of Promoter-Bound Yeast TFIID
ARTICLE DOI: 10.1038/s41467-018-07096-y OPEN Molecular structure of promoter-bound yeast TFIID Olga Kolesnikova 1,2,3,4, Adam Ben-Shem1,2,3,4, Jie Luo5, Jeff Ranish 5, Patrick Schultz 1,2,3,4 & Gabor Papai 1,2,3,4 Transcription preinitiation complex assembly on the promoters of protein encoding genes is nucleated in vivo by TFIID composed of the TATA-box Binding Protein (TBP) and 13 TBP- associate factors (Tafs) providing regulatory and chromatin binding functions. Here we present the cryo-electron microscopy structure of promoter-bound yeast TFIID at a resolu- 1234567890():,; tion better than 5 Å, except for a flexible domain. We position the crystal structures of several subunits and, in combination with cross-linking studies, describe the quaternary organization of TFIID. The compact tri lobed architecture is stabilized by a topologically closed Taf5-Taf6 tetramer. We confirm the unique subunit stoichiometry prevailing in TFIID and uncover a hexameric arrangement of Tafs containing a histone fold domain in the Twin lobe. 1 Department of Integrated Structural Biology, Equipe labellisée Ligue Contre le Cancer, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch 67404, France. 2 Centre National de la Recherche Scientifique, UMR7104, 67404 Illkirch, France. 3 Institut National de la Santé et de la Recherche Médicale, U1258, 67404 Illkirch, France. 4 Université de Strasbourg, Illkirch 67404, France. 5 Institute for Systems Biology, Seattle, WA 98109, USA. These authors contributed equally: Olga Kolesnikova, Adam Ben-Shem. Correspondence and requests for materials should be addressed to P.S. (email: [email protected]) or to G.P. -

TAF10 Complex Provides Evidence for Nuclear Holo&Ndash;TFIID Assembly from Preform
ARTICLE Received 13 Aug 2014 | Accepted 2 Dec 2014 | Published 14 Jan 2015 DOI: 10.1038/ncomms7011 OPEN Cytoplasmic TAF2–TAF8–TAF10 complex provides evidence for nuclear holo–TFIID assembly from preformed submodules Simon Trowitzsch1,2, Cristina Viola1,2, Elisabeth Scheer3, Sascha Conic3, Virginie Chavant4, Marjorie Fournier3, Gabor Papai5, Ima-Obong Ebong6, Christiane Schaffitzel1,2, Juan Zou7, Matthias Haffke1,2, Juri Rappsilber7,8, Carol V. Robinson6, Patrick Schultz5, Laszlo Tora3 & Imre Berger1,2,9 General transcription factor TFIID is a cornerstone of RNA polymerase II transcription initiation in eukaryotic cells. How human TFIID—a megadalton-sized multiprotein complex composed of the TATA-binding protein (TBP) and 13 TBP-associated factors (TAFs)— assembles into a functional transcription factor is poorly understood. Here we describe a heterotrimeric TFIID subcomplex consisting of the TAF2, TAF8 and TAF10 proteins, which assembles in the cytoplasm. Using native mass spectrometry, we define the interactions between the TAFs and uncover a central role for TAF8 in nucleating the complex. X-ray crystallography reveals a non-canonical arrangement of the TAF8–TAF10 histone fold domains. TAF2 binds to multiple motifs within the TAF8 C-terminal region, and these interactions dictate TAF2 incorporation into a core–TFIID complex that exists in the nucleus. Our results provide evidence for a stepwise assembly pathway of nuclear holo–TFIID, regulated by nuclear import of preformed cytoplasmic submodules. 1 European Molecular Biology Laboratory, Grenoble Outstation, 6 rue Jules Horowitz, 38042 Grenoble, France. 2 Unit for Virus Host-Cell Interactions, University Grenoble Alpes-EMBL-CNRS, 6 rue Jules Horowitz, 38042 Grenoble, France. 3 Cellular Signaling and Nuclear Dynamics Program, Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire, UMR 7104, INSERM U964, 1 rue Laurent Fries, 67404 Illkirch, France. -

Structure and Mechanism of the RNA Polymerase II Transcription Machinery
Downloaded from genesdev.cshlp.org on October 9, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Structure and mechanism of the RNA polymerase II transcription machinery Allison C. Schier and Dylan J. Taatjes Department of Biochemistry, University of Colorado, Boulder, Colorado 80303, USA RNA polymerase II (Pol II) transcribes all protein-coding ingly high resolution, which has rapidly advanced under- genes and many noncoding RNAs in eukaryotic genomes. standing of the molecular basis of Pol II transcription. Although Pol II is a complex, 12-subunit enzyme, it lacks Structural biology continues to transform our under- the ability to initiate transcription and cannot consistent- standing of complex biological processes because it allows ly transcribe through long DNA sequences. To execute visualization of proteins and protein complexes at or near these essential functions, an array of proteins and protein atomic-level resolution. Combined with mutagenesis and complexes interact with Pol II to regulate its activity. In functional assays, structural data can at once establish this review, we detail the structure and mechanism of how enzymes function, justify genetic links to human dis- over a dozen factors that govern Pol II initiation (e.g., ease, and drive drug discovery. In the past few decades, TFIID, TFIIH, and Mediator), pausing, and elongation workhorse techniques such as NMR and X-ray crystallog- (e.g., DSIF, NELF, PAF, and P-TEFb). The structural basis raphy have been complemented by cryoEM, cross-linking for Pol II transcription regulation has advanced rapidly mass spectrometry (CXMS), and other methods. Recent in the past decade, largely due to technological innova- improvements in data collection and imaging technolo- tions in cryoelectron microscopy. -

Supplementary Tables Supplementary Table S1. Treatment Protocols
Supplementary Tables Supplementary Table S1. Treatment protocols pediatric AML patients. Number of Treatment patients ADE-based regimen 64 ADE with bortezomib 14 ADE with mylotarg 6 APL-like treatment 2 TMD-like treatment 1 Relapse therapy 2 Misc treatment/novel agents 5 Unknown 2 Supplemental Table S2: Patient characteristics for the 73 pediatric acute lymphoblastic leukemia patients Characteristics N, % Number of cases 73 ALL subtype Pre-B ALL 57 (78) T-ALL 16 (22) Age, y, median (range) 7.3 (0.2-18.0) Gender Male 40 (55) Female 33 (45) Declared Ethnicity Caucasian 61 (84) Hispanic 44 (72) Non-Hispanic 17 (28) Black American 6 (8) Asian 4 (5) Mixed 2 (3) Hispanic 1 (1) ‘‘SNP’’ Ethnicity European 9 (12) African 6 (8) American Indian 38 (52) Asian 1 (1) Not done 19 (26) Cytogenetics Favorable 15 (21) Intermediate 42 (58) Unfavorable 15 (21) Unknown 1 (1) Risk Group Low Risk 4 (5) Standard/ Intermediate Risk 29 (40) High/Very High Risk 40 (55) CNS status CNS-1 46 (63) CNS-2 20 (27) CNS-3 6 (8) Unknown 2 (3) Response Complete remission 67 (92) Resistant 4 (5) Fail 2 (3) Alive 63 (86) Supplementary Table S3. The table shows the ‘‘Rosetta Stone’’ of the antibody and protein nomenclature, and the R2 for the antibody validation and the primary and secondary antibody dilutions. Secondary, all antibodies that were used in combination with information about the manufacturer of each antibody, the antibody source, and catalog number are listed. Functional Protein Name Rosetta Stone RPPA Staining Details Category Functional Effect Common Name RPPA Antibody Huge Name (added MiMI Antibody R2, WB vs. -

BET Family Members Bdf1/2 Modulate Global Transcription Initiation and Elongation in Saccharomyces Cerevisiae Rafal Donczew*, Steven Hahn*
RESEARCH ARTICLE BET family members Bdf1/2 modulate global transcription initiation and elongation in Saccharomyces cerevisiae Rafal Donczew*, Steven Hahn* Fred Hutchinson Cancer Research Center, Division of Basic Sciences, Seattle, United States Abstract Human bromodomain and extra-terminal domain (BET) family members are promising targets for therapy of cancer and immunoinflammatory diseases, but their mechanisms of action and functional redundancies are poorly understood. Bdf1/2, yeast homologues of the human BET factors, were previously proposed to target transcription factor TFIID to acetylated histone H4, analogous to bromodomains that are present within the largest subunit of metazoan TFIID. We investigated the genome-wide roles of Bdf1/2 and found that their important contributions to transcription extend beyond TFIID function as transcription of many genes is more sensitive to Bdf1/2 than to TFIID depletion. Bdf1/2 co-occupy the majority of yeast promoters and affect preinitiation complex formation through recruitment of TFIID, Mediator, and basal transcription factors to chromatin. Surprisingly, we discovered that hypersensitivity of genes to Bdf1/2 depletion results from combined defects in transcription initiation and early elongation, a striking functional similarity to human BET proteins, most notably Brd4. Our results establish Bdf1/2 as critical for yeast transcription and provide important mechanistic insights into the function of BET proteins in all eukaryotes. *For correspondence: [email protected] (RD); Introduction [email protected] (SH) Bromodomains (BDs) are reader modules that allow protein targeting to chromatin via interactions with acetylated histone tails. BD-containing factors are usually involved in gene transcription, and Competing interests: The their deregulation has been implicated in a spectrum of cancers and immunoinflammatory and neu- authors declare that no rological conditions (Fujisawa and Filippakopoulos, 2017; Wang et al., 2021). -
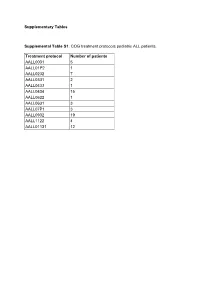
Supplementary Tables Supplemental Table S1. COG Treatment Protocols
Supplementary Tables Supplemental Table S1. COG treatment protocols pediatric ALL patients. Treatment protocol Number of patients AALL0031 5 AALL01P2 1 AALL0232 7 AALL0331 2 AALL0433 1 AALL0434 15 AALL0622 1 AALL0631 3 AALL07P1 3 AALL0932 19 AALL1122 4 AALL01131 12 Supplementary Table S2. This table contains the “Rosetta Stone” for the antibody and protein nomenclature (HUGO, MiMI, GeneCards) together with the RPPA staining details, including the primary and secondary antibody dilutions, the R2 scores For antibody validation, catalog number and the antibody source. Functional Protein Name Rosetta Stone RPPA Staining Details Category Functional Effect Common Name RPPA Antibody Huge Name (added MiMI Antibody R2, WB vs. Antibody 2nd Ab Full Name/Description from GeneCards of Mfg Claimed Manufacturer Catalog# Functional Group Name with PTMs) Name Source RPPA Dilution dilution Phosphorylation Target AKT1 AKT1 AKT1 v-akt murine thymoma viral oncogene homolog 1 AKT1 Cell Signaling 9272 Rabbit > 0.7 200 15000 PI3KAKT AKT1/AKT2/AKT3 AKT1_2_3.pS473 AKT1 v-akt murine thymoma viral oncogene homolog 1 Activation AKT-P473(Ser) Cell Signaling 9271 Rabbit > 0.7 150 20000 PI3KAKT Phospho Ser473 AKT1/AKT2/AKT3 AKT1_2_3.pT308 AKT1 v-akt murine thymoma viral oncogene homolog 1 Activation AKT-P308(Thr) Cell Signaling 9275 Rabbit > 0.7 200 20000 PI3KAKT Phospho Thr308 ASNS ASNS ASNS asparagine synthetase (glutamine-hydrolyzing) ASNS Sigma HPA029318 Rabbit 0.5-0.7 800 20000 Metabolic ATF3 ATF3 ATF3 activating transcription factor 3 ATF3 Abcam ab87213 Rabbit -

TAF4 Takes Flight
COMMENTARY TAF4 takes flight Michael T. Marr II1 Department of Biology and Rosenstiel Basic Medical Sciences Research Center, Brandeis University, Waltham, MA 02454 he eukaryotic mRNA transcrip- from yeast to man, but the region of ETO-TAFH domain was required for tion machinery is exceedingly conservation is limited to a histone H2A activation of the wingless target gene complex, perhaps reflecting the homology region at the carboxyl termi- naked cuticle (nkd) both in cultured requirement for response to nus of the protein (Fig. 1A). The meta- cells and in larval imaginal discs. They Tdiverse environmental and developmen- zoan homologues of TAF4 contain an further showed that this domain directly tal signals. Some of the machinery is extended amino terminus with two addi- interacts with the amino terminus of the common to all mRNA genes and in- tional conserved regions: a glutamine- pygopus, a dedicated transcription factor cludes RNA polymerase II (Pol II) and rich region and a more recently defined for wingless signaling. Interestingly, the a set of general transcription factors ETO-TAFH domain. amino terminus of pygopus has previ- (GTFs) (1). Signal-specific gene expres- The glutamine-rich region was one of ously been defined as absolutely neces- sion patterns are defined by sequence- the first coactivator domains of TFIID sary for Wg/Wnt signaling (14). These specific DNA-binding proteins (activa- to be defined (8). It interacts with a findings place TAF4 at the end of a tors) that bind cognate sites in the number of activators including Sp1 and chain of protein–protein interactions promoter and enhancers of target genes. -

NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-7-2018 Decipher Mechanisms by which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer Jairo Ramos [email protected] Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Clinical Epidemiology Commons Recommended Citation Ramos, Jairo, "Decipher Mechanisms by which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer" (2018). FIU Electronic Theses and Dissertations. 3872. https://digitalcommons.fiu.edu/etd/3872 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida DECIPHER MECHANISMS BY WHICH NUCLEAR RESPIRATORY FACTOR ONE (NRF1) COORDINATES CHANGES IN THE TRANSCRIPTIONAL AND CHROMATIN LANDSCAPE AFFECTING DEVELOPMENT AND PROGRESSION OF INVASIVE BREAST CANCER A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in PUBLIC HEALTH by Jairo Ramos 2018 To: Dean Tomás R. Guilarte Robert Stempel College of Public Health and Social Work This dissertation, Written by Jairo Ramos, and entitled Decipher Mechanisms by Which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer, having been approved in respect to style and intellectual content, is referred to you for judgment. -

Short- and Long-Term Impact of Hyperoxia on the Blood and Retinal Cells’ Transcriptome in a Mouse Model of Oxygen- Induced Retinopathy
www.nature.com/pr BASIC SCIENCE ARTICLE OPEN Short- and long-term impact of hyperoxia on the blood and retinal cells’ transcriptome in a mouse model of oxygen- induced retinopathy Magdalena Zasada1, Anna Madetko-Talowska2, Cecilie Revhaug3,4, Anne Gro W. Rognlien3,4, Lars O. Baumbusch3,Teofila Książek2, Katarzyna Szewczyk2, Agnieszka Grabowska2, Miroslaw Bik-Multanowski2, Jacek Józef Pietrzyk1, Przemko Kwinta1 and Ola Didrik Saugstad3,4 BACKGROUND: We aimed to identify global blood and retinal gene expression patterns in murine oxygen-induced retinopathy (OIR), a common model of retinopathy of prematurity, which may allow better understanding of the pathogenesis of this severe ocular prematurity complication and identification of potential blood biomarkers. METHODS: A total of 120 C57BL/6J mice were randomly divided into an OIR group, in which 7-day-old pups were maintained in 75% oxygen for 5 days, or a control group. RNA was extracted from the whole-blood mononuclear cells and retinal cells on days 12, 17, and 28. Gene expression in the RNA samples was evaluated with mouse gene expression microarrays. RESULTS: There were 38, 1370 and 111 genes, the expression of which differed between the OIR and control retinas on days 12, 17, and 28, respectively. Gene expression in the blood mononuclear cells was significantly altered only on day 17. Deptor and Nol4 genes showed reduced expression both in the blood and retinal cells on day 17. 1234567890();,: CONCLUSION: There are sustained marked changes in the global pattern of gene expression in the OIR mice retinas. An altered expression of Deptor and Nol4 genes in the blood mononuclear cells requires further investigation as they may indicate retinal neovascularization. -

Granulocyte Colony-Stimulating Factor-Induced TAF9 Modulates P53-TRIAP1-CASP3 Axis to Prevent Retinal Ganglion Cell Death After Optic Nerve Ischemia
Granulocyte Colony-Stimulating Factor-Induced TAF9 Modulates P53-TRIAP1-CASP3 Axis to Prevent Retinal Ganglion Cell Death after Optic Nerve Ischemia Yao-Tseng Wen Buddhist Tzu Chi General Hospital Hualien: Hualien Tzu Chi Hospital Keh-Liang Lin Chung Shan Medical University Hospital Chin-Te Huang Tzu Chi University Rong Kung Tsai ( [email protected] ) Hualien Tzu Chi Hospital https://orcid.org/0000-0001-8760-5739 Research article Keywords: Granulocyte colony-stimulating factor, Rat anterior ischemic optic neuropathy model, Retinal ganglion cell, TBP associated factor 9, TP53, TRIAP1 Posted Date: January 6th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-138908/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/25 Abstract Background Optic nerve head (ONH) infarct can result in progressive retinal ganglion cell (RGC) death. Some evidences indicated that the granulocyte colony-stimulating factor (GCSF) provides positive effects against ischemic damage on RGCs. However, protective mechanisms of the GCSF after ONH infarct are complex and remain unclear. Methods To investigate the complex mechanisms, the transcriptome proles of the GCSF-treated retinas were examined using microarray technology. The retinal mRNA samples on days 3 and 7 post rat anterior ischemic optic neuropathy model (rAION) were analyzed by microarray and bioinformatics analyses. To evaluate the TAF9 function in RGC apoptosis, GCSF plus TAF9 siRNA-treated rats were evaluated using retrograde labeling with FluoroGold assay, TUNEL assay, and Western blotting in a rAION. Results GCSF treatment inuenced 3101 genes and 3332 genes on days 3 and 7 post rAION, respectively. -

The Relationship Between Long-Range Chromatin Occupancy and Polymerization of the Drosophila ETS Family Transcriptional Repressor Yan
INVESTIGATION The Relationship Between Long-Range Chromatin Occupancy and Polymerization of the Drosophila ETS Family Transcriptional Repressor Yan Jemma L. Webber,*,1 Jie Zhang,*,†,1 Lauren Cote,* Pavithra Vivekanand,*,2 Xiaochun Ni,‡,§ Jie Zhou,§ Nicolas Nègre,**,3 Richard W. Carthew,†† Kevin P. White,‡,§,** and Ilaria Rebay*,†,4 *Ben May Department for Cancer Research, †Committee on Cancer Biology, ‡Department of Ecology and Evolution, §Department of Human Genetics, and **Institute for Genomics and Systems Biology, University of Chicago, Chicago, Illinois 60637, and ††Department of Molecular Biosciences, Northwestern University, Evanston, Illinois 60208 ABSTRACT ETS family transcription factors are evolutionarily conserved downstream effectors of Ras/MAPK signaling with critical roles in development and cancer. In Drosophila, the ETS repressor Yan regulates cell proliferation and differentiation in a variety of tissues; however, the mechanisms of Yan-mediated repression are not well understood and only a few direct target genes have been identified. Yan, like its human ortholog TEL1, self-associates through an N-terminal sterile a-motif (SAM), leading to speculation that Yan/TEL1 polymers may spread along chromatin to form large repressive domains. To test this hypothesis, we created a monomeric form of Yan by recombineering a point mutation that blocks SAM-mediated self-association into the yan genomic locus and compared its genome-wide chromatin occupancy profile to that of endogenous wild-type Yan. Consistent with the spreading model predictions, wild-type Yan-bound regions span multiple kilobases. Extended occupancy patterns appear most prominent at genes encoding crucial developmental regulators and signaling molecules and are highly conserved between Drosophila melanogaster and D. virilis, suggest- ing functional relevance.