Core 1..152 Hansard (PRISM::Advent3b2 7.50)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Core 1..196 Hansard (PRISM::Advent3b2 10.50)
CANADA House of Commons Debates VOLUME 144 Ï NUMBER 025 Ï 2nd SESSION Ï 40th PARLIAMENT OFFICIAL REPORT (HANSARD) Friday, March 6, 2009 Speaker: The Honourable Peter Milliken CONTENTS (Table of Contents appears at back of this issue.) Also available on the Parliament of Canada Web Site at the following address: http://www.parl.gc.ca 1393 HOUSE OF COMMONS Friday, March 6, 2009 The House met at 10 a.m. Some hon. members: Yes. The Speaker: The House has heard the terms of the motion. Is it the pleasure of the House to adopt the motion? Prayers Some hon. members: Agreed. (Motion agreed to) GOVERNMENT ORDERS Mr. Mark Warawa (Parliamentary Secretary to the Minister of the Environment, CPC) moved that Bill C-17, An Act to Ï (1005) recognize Beechwood Cemetery as the national cemetery of Canada, [English] be read the second time and referred to the Standing Committee on Environment and Sustainable Development. NATIONAL CEMETERY OF CANADA ACT He said: Mr. Speaker, I would like to begin by seeking unanimous Hon. Jay Hill (Leader of the Government in the House of consent to share my time. Commons, CPC): Mr. Speaker, momentarily, I will be proposing a motion by unanimous consent to expedite passage through the The Speaker: Does the hon. member have unanimous consent to House of an important new bill, An Act to recognize Beechwood share his time? Cemetery as the national cemetery of Canada. However, before I Some hon. members: Agreed. propose my motion, which has been agreed to in advance by all parties, I would like to take a quick moment to thank my colleagues Mr. -

Association Newsletter 2007
408 “Goose” Squadron Association Newsletter 2007 Inside this issue: National Chairman 1 Missing In Action 12 Squadron Activities 2 Membership 16 Change of Command 5 Remembering The Fallen 17 Commanding Officer 7 Final Fly Past 21 Squadron Chief 8 Memories From the Past 22 Mother Goose 10 Letters and Requests 30 W/C Ferris DFC From the Chairman The famous 408 “Goose” Squadron has entered into its 66th year of service to Canada. Looking back on our accomplishments can only instill a sense of pride for all who have served under the “For Freedom” banner. Our contribution to the his- tory of our country has been significant and will continue with the next generation of airmen and airwomen. Sixty-six years is a long time for any particular entity and almost unbelievable for an operational unit such as ours. Whether currently flying in war torn Afghanistan, the vast wilderness of northern Canada during the 50’s and 60’s or the dark dangerous skies of Fortress Europe in WWII we continue to meet every challenge with the same fortitude and resolve. 408 Squadron remains proud, strong and ready! The word “hectic” would probably be the best way to describe the actions around 408 Squadron since the last newsletter. A considerable amount of training and preparations have been undertaken to evaluate and accredit the squadron to a high readiness state. The units under the control of 1 Wing Headquarters, based in Kingston, rotate readiness levels so that an individual unit is always maintained and prepared for whatever contingency that may arise. -

Gloucester Street Names Including Vanier, Rockcliffe, and East and South Ottawa
Gloucester Street Names Including Vanier, Rockcliffe, and East and South Ottawa Updated March 8, 2021 Do you know the history behind a street name not on the list? Please contact us at [email protected] with the details. • - The Gloucester Historical Society wishes to thank others for sharing their research on street names including: o Société franco-ontarienne du patrimoine et de l’histoire d’Orléans for Orléans street names https://www.sfopho.com o The Hunt Club Community Association for Hunt Club street names https://hunt-club.ca/ and particularly John Sankey http://johnsankey.ca/name.html o Vanier Museoparc and Léo Paquette for Vanier street names https://museoparc.ca/en/ Neighbourhood Street Name Themes Neighbourhood Theme Details Examples Alta Vista American States The portion of Connecticut, Michigan, Urbandale Acres Illinois, Virginia, others closest to Heron Road Blackburn Hamlet Streets named with Eastpark, Southpark, ‘Park’ Glen Park, many others Blossom Park National Research Queensdale Village Maass, Parkin, Council scientists (Queensdale and Stedman Albion) on former Metcalfe Road Field Station site (Radar research) Eastway Gardens Alphabeted streets Avenue K, L, N to U Hunt Club Castles The Chateaus of Hunt Buckingham, Club near Riverside Chatsworth, Drive Cheltenham, Chambord, Cardiff, Versailles Hunt Club Entertainers West part of Hunt Club Paul Anka, Rich Little, Dean Martin, Boone Hunt Club Finnish Municipalities The first section of Tapiola, Tammela, Greenboro built near Rastila, Somero, Johnston Road. -

It June 17/04 Copy
Strait of Georgia VOTE! Monday Every Second Thursday & Online ‘24/7’ at islandtides.com June 28th Canadian Publications Mail Product Volume 16 Number 11 Your Coastal Community Newspaper June 17–June 30, 2004 Sales Agreement Nº 40020421 Tide tables 2 Saturna notes 3 Letters 4 What’s on? 5 Advance poll 7 Bulletin board 11 Closely watched votes: Saanich–Gulf Islands race may be critical The electoral battle for the federal parliamentary seat in the Saanich-Gulf Islands riding has drawn national attention. It’s not just that the race has attracted four strong candidates from the Conservative, Liberal, NDP and Photo: Brian Haller Green parties. It’s that the riding has the Haying at Deacon Vale Farm, one of Mayne Island’s fourteen farms. Mayne’s Farmers Market begins in July. best chance in Canada to actually elect a Green Party member. The Green Party is running candidates in all 308 federal ridings in Elections, then and now ~ Opinion by Christa Grace-Warrick & Patrick Brown this election, the first time it has done this. Nationally, polls have put the Green t has been said that generals are always fighting the previous war. It in debate and more free votes. If this is the case, the other parties will vote at a couple of percentage points. might also be said that politicians are always fighting the last election, eventually have to do the same. The polls (as we write) indicate the possibility But the Greens have plenty of room to Iand that the media are always reporting the last election. -
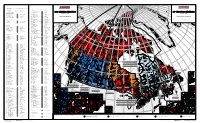
Map of Canada, Official Results of the 38Th General Election – PDF Format
2 5 3 2 a CANDIDATES ELECTED / CANDIDATS ÉLUS Se 6 ln ln A nco co C Li in R L E ELECTORAL DISTRICT PARTY ELECTED CANDIDATE ELECTED de ELECTORAL DISTRICT PARTY ELECTED CANDIDATE ELECTED C er O T S M CIRCONSCRIPTION PARTI ÉLU CANDIDAT ÉLU C I bia C D um CIRCONSCRIPTION PARTI ÉLU CANDIDAT ÉLU É ol C A O N C t C A H Aler 35050 Mississauga South / Mississauga-Sud Paul John Mark Szabo N E !( e A N L T 35051 Mississauga--Streetsville Wajid Khan A S E 38th GENERAL ELECTION R B 38 ÉLECTION GÉNÉRALE C I NEWFOUNDLAND AND LABRADOR 35052 Nepean--Carleton Pierre Poilievre T A I S Q Phillip TERRE-NEUVE-ET-LABRADOR 35053 Newmarket--Aurora Belinda Stronach U H I s In June 28, 2004 E T L 28 juin, 2004 É 35054 Niagara Falls Hon. / L'hon. Rob Nicholson E - 10001 Avalon Hon. / L'hon. R. John Efford B E 35055 Niagara West--Glanbrook Dean Allison A N 10002 Bonavista--Exploits Scott Simms I Z Niagara-Ouest--Glanbrook E I L R N D 10003 Humber--St. Barbe--Baie Verte Hon. / L'hon. Gerry Byrne a 35056 Nickel Belt Raymond Bonin E A n L N 10004 Labrador Lawrence David O'Brien s 35057 Nipissing--Timiskaming Anthony Rota e N E l n e S A o d E 10005 Random--Burin--St. George's Bill Matthews E n u F D P n d ely E n Gre 35058 Northumberland--Quinte West Paul Macklin e t a s L S i U a R h A E XEL e RÉSULTATS OFFICIELS 10006 St. -

Public Consultation Report No. 2 December 2013
Former Rockcliffe Airbase Community Design Plan Ottawa ON Public Consultation Report No. 2 December 2013 TABLE OF CONTENTS Page 1. Introduction 2 2. Overall Consultation Objectives 2 3. Public Notifications 3 4. Public Advisory Group 4 5. Other Consultation Meetings 5 A. Phase 1 5 B. Phase 2 6 6. Summary of Major Consultation Events 7 A. Phase 1 - November 26, 2012 7 B. Phase 2 – May 25, 2013 9 7. Next Steps: Phase 3 - Community Design Plan (CDP) 16 APPENDICES APPENDIX 1: Public Notices 18 1A – Phase 1 - November 26, 2012 19 1B – Phase 2 - May 25, 2013 20 APPENDIX 2: Public Advisory Group – Meeting Notes 21 2A – Phase 1 22 2B – Phase 2 28 APPENDIX 3: Ideas Fair – November 26, 2012 48 3A - Meeting Notes 49 3B - Summary of Comments Received 60 3C – Questionnaire 63 APPENDIX 4: Interactive Public Workshop and Open House – May 25, 2013 64 4A – Agenda 65 4B - Summary of Comments 66 4C - Evaluation Sheet 70 APPENDIX 5: Guiding Design Vision and Principles 72 Page | 1 1 - Introduction In October 2012, Canada Lands Company commenced an integrated Planning Act and Environmental Assessment Act process for the lands formerly known as CFB Rockcliffe. The purpose of the project is to prepare a Community Design Plan (CDP) for approval by the City of Ottawa. A key component of the CDP process is the coordination and integration of the public consultation for the CDP, including the Planning Act requirements for an implementing Official Plan Amendment and the requirements of the Municipal Class Environmental Assessment for related environmental and infrastructure projects. -

The History of Canadian Military Communications and Electronics
9900 YYEEAARRSS AANNDD CCOOUUNNTTIINNGG THE HISTORY OF CANADIAN MILITARY COMMUNICATIONS AND ELECTRONICS Captain John A. MacKenzie Canadian Forces Communications and Electronics MUSEUM UPDATED: 25 September, 1995 THE HISTORY OF THE COMMUNICATIONS AND ELECTRONICS BRANCH CONTENTS CHAPTER 1 IN THE BEGINNING 1867 - 1913. Early communications requirements and activities, the Yukon Telegraph Service, the Canadian Engineers Signal Service and its development. CHAPTER 2 THE BIRTH OF THE CANADIAN SIGNALLING CORPS. Formation of the Canadian Signalling Corps and developments from 1903 to 1913, the lead up to World War One. CHAPTER 3 WORLD WAR ONE 1914 - 1918. The military communications events and important dates during the war. CHAPTER 4 BETWEEN THE WARS 1919 - 1939. Evolution of early military communications, the North West Territories and Yukon Radio System, the Forestry Service, Mapping and Charting, the birth of RCAF Signals and early RCN shore stations. Preparations for war. CHAPTER 5 WORLD WAR TWO 1939 - 1945. Canadian communications and important events during the war. CHAPTER 6 THE COLD WARRIORS 1946 - 1989. North Atlantic Treaty Organization participation, United Nations operations and Canadian communications development since World War Two, integration of the Canadian Forces, the new C & E Branch. CHAPTER 7 TOWARD A NEW WORLD (DIS)ORDER 1989 - . The collapse of the Warsaw Pact, Canadian military downsizing as part of the "Peace Dividend", peace keeping and peace making in a destabilized world. ANNEX A PEACEKEEPING MISSIONS Summary of United Nations and other related peace keeping missions. ANNEX B DIEPPE RAID PARTICIPANTS Summary of Signals participants in the raid of 19 August 1942. ANNEX C WORLD WAR II GROUND RADAR Early Developments. -

Core 1..132 Hansard (PRISM::Advent3b2 6.50.00)
CANADA House of Commons Debates VOLUME 137 Ï NUMBER 167 Ï 1st SESSION Ï 37th PARLIAMENT OFFICIAL REPORT (HANSARD) Friday, April 12, 2002 Speaker: The Honourable Peter Milliken CONTENTS (Table of Contents appears at back of this issue.) All parliamentary publications are available on the ``Parliamentary Internet Parlementaire´´ at the following address: http://www.parl.gc.ca 10343 HOUSE OF COMMONS Friday, April 12, 2002 The House met at 10 a.m. fish products, to sell or otherwise dispose of these products, and to make deficiency payments to producers. The intent of the act was to Prayers protect fishermen against sharp declines in prices and consequent loss of income due to causes beyond the control of fishermen or the fishing industry. GOVERNMENT ORDERS The board has not undertaken any significant price support Ï (1000) activities since 1982 except for the purchase of fish as food aid for [English] distribution by CIDA. AN ACT TO AMEND CERTAIN ACTS AND INSTRUMENTS AND TO REPEAL THE FISHERIES PRICES SUPPORT ACT Bill C-43 can be considered a hybrid of the Miscellaneous Statute Law Amendment Act. Bill C-43 contains a number of provisions The House resumed from December 7 consideration of the omitted from the draft of the Miscellaneous Statute Law Amendment motion that Bill C-43, an act to amend certain Acts and instruments Act, MSLA, Bill C-40. The miscellaneous statute law amendment and to repeal the Fisheries Prices Support Act, be read the third time program was initiated in 1975 to allow for minor, non-controversial and passed. amendments to federal statutes in an omnibus bill. -

Unplugging the Dirty Energy Economy / Ii
Polaris Institute, June 2015 The Polaris Institute is a public interest research organization based in Canada. Since 1997 Polaris has been dedicated to developing tools and strategies to take action on major public policy issues, including the corporate power that lies behind public policy making, on issues of energy security, water rights, climate change, green economy and global trade. Acknowledgements This Profile was researched and written by Mehreen Amani Khalfan, with additional research from Richard Girard, Daniel Cayley-Daoust, Erin Callary, Alexandra Bly and Brianna Aird. Special thanks to Heather Milton-Lightening and Clayton Thomas-Muller for their contributions. Cover design by Spencer Mann. This project was made possible through generous support from the European Climate Fund Polaris Institute 180 Metcalfe Street, Suite 500 Ottawa, ON K2P 1P5 Phone: 613-237-1717 Fax: 613-237-3359 Email: [email protected] www.polarisinstitute.org i / Polaris Institute Table of Contents SUMMARY ............................................................................................................................................................. 1 INTRODUCTION ..................................................................................................................................................... 4 CHAPTER 1 - ORGANIZATIONAL PROFILE ............................................................................................................... 6 1.1 TRANSCANADA’S BUSINESS STRUCTURE AND OPERATIONS .............................................................................................. -

The Nstar Chronicle
The NStar Chronicle The Project North Star Association of Canada Volume 16| Issue 1| March 2020 Editor’s Notes Roger Button Putting out this edition of the Chronicle has been an example of great co-operation from members and non-members alike. Thanks are owed to a number of people. Our member Gary Whitten was instrumental in getting the necessary permission from Air Force Magazine to publish the "Diversion to Alert" article. In addition thanks go out to the other contributors, namely Richard Lodge, Garry Dupont, Neil Raynor, and Réjean Demers. A special thanks to Bruce Gemmill who took the lead in putting together the progress report, which can be found in the Conservator’s Corner section. Finally, I would like to thank Drew Hodge our stalwart publisher of this edition. We do appreciate receiving feedback from our readers including comments on the articles or suggestions for new articles. We are always on the lookout for content for the Chronicle so please do not hesitate to bring forward any ideas that you might have. As noted in the Conservator’s Corner article the restoration of engine number 4 has been completed. Our next edition will focus on the engine restoration work so if you have any material or comments specific to that aspect of the restoration please let us know. Enjoy. PNSAC Contents of this issue: Diversion to Alert . 6 A Personal Journey in Support of Homeless Editor’s Notes . 2 Veterans in Canada . 13 Notes from the President . 2 Conservator’s Corner . 3 Calendar of Events . 14 Our Members . -

Historic Gloucester Volume 11 No 3.Pub
Historic Gloucester Newsletter of the GLOUCESTER HISTORICAL SOCIETY Www.gloucesterhistory.com VOLUME 11, NO 3 Fall 2010 , Montreal and Russell Roads Tollhouse Historic Gloucester - 2 - Vol 11, No 3, 2010 From the Editor’s Desk………………………………………………………….. Joan Scott 3 A Brief History of The Rockcliffe Air Station……………………….………… Robert Serré 4 Pioneer Settlers’s Difficulties ……………………...………………………….. Joan Scott 6 Disappearing Homes…………………………………………………………….. 7 Publications………………………………………………………………………. 8 Membership Form……………………………………………………………….. 10 THE GLOUCESTER HISTORICAL SOCIETY IS HAPPY TO ANNOUNCE THAT ITS HISTORY ROOM IS OPEN TO THE PUBLIC EACH THURSDAY FROM 10:00 A.M. to 3:00 P.M. BETWEEN MAY 6 AND OCTOBER 28, 2010 LOCATION: 4550B BANK STREET (AT LEITRIM ROAD) FOR MORE INFORMATON Contact Robert Serré at 613-749-0607 // [email protected] Cover Photo: This circa 1915 photo is of a tollhouse that was on the corner of Montreal and Russell Roads. The tollhouse was owned by the Ottawa, Montreal and Russell Consolidated Road Company. Gatekeeper William McPhail stands at the door. Tolls were used by municipalities to raise funds for civic projects. The toll for a horse was 10 cents, for two horses 16 cents and for a car 25 cents. Historic Gloucester is published by The Gloucester Historical Society. It is intended as a Newsletter to members of the Society to provide interesting articles on Gloucester’s past and to keep them informed of new acquisitions by the Museum, publications available, upcoming events and other items of general interest. Comments and suggestions regarding the Newsletter are always welcome. Gloucester Historical Society gratefully acknowledges the financial support of the City of Ottawa. -

Bioenergy Policy and Regulation in Canada – November 2009
Timeline Bioenergy Policy and Regulation in Canada Last Update: November 2009 Principal Researcher: Alin Charrière Master’s Student, School of Public Policy and Administration Series Editor: Marc Saner, Director of Research, Regulatory Governance Initiative Explanatory Note This timeline outlines important events related to bioenergy policy and regulation in Canada with an emphasis on biofuels and developments since 2000. For the purposes of this timeline, Bioenergy refers to various forms of renewable energy produced from biomass, such as biofuels, biopower and bioheat. The research team has made an effort to highlight events relevant to the forestry sector. For background purposes, the research team has also chosen to include broader developments in energy policy as well as significant events outside of Canada where deemed appropriate. Please help us keep this timeline accurate and up-to-date by providing comments to [email protected]. Acknowledgments The following individuals contributed to the development of this timeline: Jeffery Cottes, Holly Mitchell, Dr. Jennifer Pelley, Prof. Myron Smith and Prof. David Miller. The Regulatory Governance Initiative also gratefully acknowledges financial support from the Forest Products Association of Canada (FPAC) and the Canada School of Public Service, without which this work would not have been possible. This work is licensed under a Creative Commons Attribution – Non-commercial – No Derivatives License. To view this license, visit (www.creativecommons.org/licenses/by-nc-nd/2.5/). For re-use or distribution, please include this copyright notice. © 2009 Regulatory Governance Initiative, Carleton University Timeline – Bioenergy Policy and Regulation in Canada Event Who When Description Relates To Early vehicles use a Various Early 1900s Early vehicles are powered by a diversity of fuels such as ethanol, diesel, History of wide variety of biofuels butanol, peanut oil, fuels obtained through pyrolysis or gasification, etc.