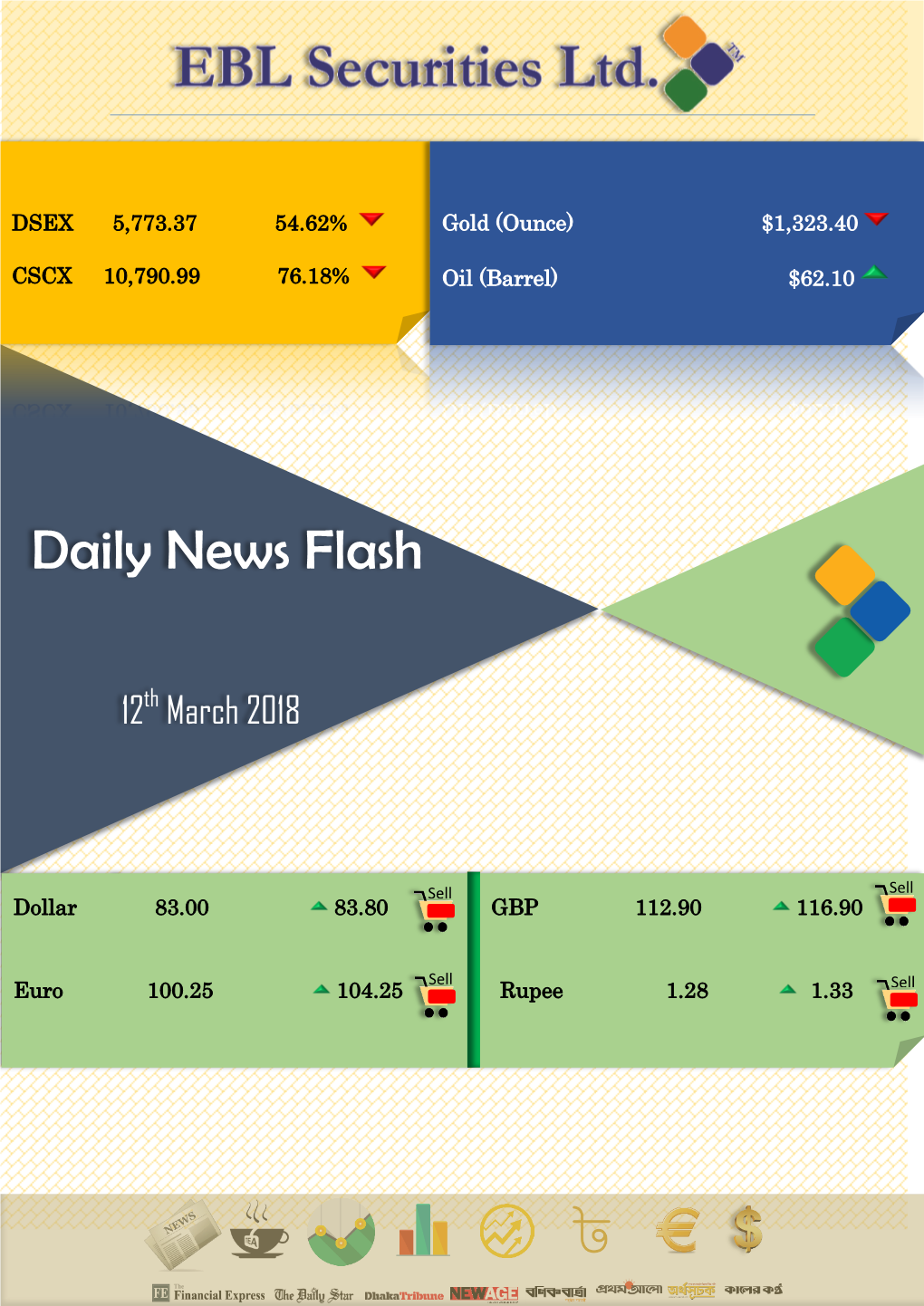
Daily News Flash
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Korea Innovative PHAR
2020 This brochure illustrates a project promoted by Korean pharmaceutical companies and with support from the Korean government, to enter global markets. You’ll meet the Korean pharmaceutical industry as a reliable partner for global collaboration. Contents 02 Current Status of Korean Pharmaceutical Market 08 Introduction of Korean Innovative Pharmaceutical Company Certification System 10 Information of Korean Innovative Pharmaceutical Company 55 Supplement Current Status of Korean Pharmaceutical Market Current Status of (Market size) Korean pharmaceutical market passed the $21 billion mark in 2018. Its Korean Pharmaceutical Market growth has been driven by leading Korean companies, which have been releasing new global drugs and achieving technology exports. Pharmaceuticals exports have increased at a CAGR of 17.9% for the past five years (2014-2018), fueled especially by increases in Korean drugs entering into global markets. Current status of Korean pharmaceutical market (unit: million dollar, %) Year-on-year CAGR Category 2014 2015 2016 2017 2018 growth rate (’14~’18) Market size 18,396 16,963 18,712 19,508 21,004 7.70% 3.40% Production 15,593 14,991 16,198 18,000 19,176 6.50% 5.30% Exports 2,416 2,946 3,119 4,069 4,673 14.80% 17.90% Imports 5,219 4,918 5,633 5,577 6,501 16.60% 5.60% Trade balance △2,803 △1,972 △2,514 △1,508 △1,828 △21.2% △10.1% * Source: Press release by the Ministry of Food and Drug Safety (July 29 2019) • The 2018 sales revenue of the listed drug companies (125) is $18,172 million in total (7.5% up from the previous year), with Top 10’s revenue at $7,814 million (8.6%↑). -

Employment of Veterinarians
CONTENTS TOPIC PAGE Foreword 1 Summary of Project Since 1965 5 Veterinary Medicine in Kenya The Livestock Industry 8 Employment of Veterinarians 11 Livestock Diseases 14 Zoonoses 16 Research, Extension and Continuing Education 17 Operational Plan, Contract AID/afr-790 21 Scope of Work 22 Contract Staff 22 History of the Faculty of Veterinary Medicine 24 Beginning of Colorado State University Program of Technical Assistance 27 Faculty of Veterinary Medicine Structure 29 Undergraduate Program of Study 29 Structure of Curriculum 30 Courses of Study 30 Examination Sche2me 31 Outlines of Courses 33 Postgraduate Programs of Study 39 Faculty of Veterinary Medicine, Departmental Structure Clinical Studies 41 Pathology and Microbiology 43 Anatomy and Histology 45 Animal Physiology 46 Public Health, Pharmacology and Toxicology 47 Kenyanization of the Faculty 48 Government of Kenya Contribution to the Project 49 Professional Staff Supplied by C.S.U. Field Staff in Nairobi 54 Campus Coordinators 60 Administrative Staff 60 Recruitment of Field Staff 62 Publications by C.S.U. Field Staff 64 Publications by Counterparts 66 Unpublished Research 68 Post Graduate Courses 69 Graduate Student Supervision 70 International Meetings 72 External Examiners from C.S.U. 73 Participant and Counterpart Training Summary 74 Distribution of Participants in U.S. Universities 74 Selection of Participants 75 Support of Participants Upon Return-to Kenya 77 Participants 78 Counterpart Training 85 Final Reports of Field Staff Parasitology, Dr. A. E. Sollod 87 Clinical Studies, -

WHO Monographs on Selected Medicinal Plants. Volume 3
WHO monographs on WHO monographs WHO monographs on WHO published Volume 1 of the WHO monographs on selected medicinal plants, containing 28 monographs, in 1999, and Volume 2 including 30 monographs in 2002. This third volume contains selected an additional collection of 32 monographs describing the quality control and use of selected medicinal plants. medicinal Each monograph contains two parts, the first of which provides plants selected medicinal plants pharmacopoeial summaries for quality assurance purposes, including botanical features, identity tests, purity requirements, Volume 3 chemical assays and major chemical constituents. The second part, drawing on an extensive review of scientific research, describes the clinical applications of the plant material, with detailed pharmacological information and sections on contraindications, warnings, precautions, adverse reactions and dosage. Also included are two cumulative indexes to the three volumes. The WHO monographs on selected medicinal plants aim to provide scientific information on the safety, efficacy, and quality control of widely used medicinal plants; provide models to assist Member States in developing their own monographs or formularies for these and other herbal medicines; and facilitate information exchange among Member States. WHO monographs, however, are Volume 3 Volume not pharmacopoeial monographs, rather they are comprehensive scientific references for drug regulatory authorities, physicians, traditional health practitioners, pharmacists, manufacturers, research scientists -

Korea Innovative PHARMACEUTICAL Company Potential of Pharmaceutical Industry in Korea
2018 Korea Innovative PHARMACEUTICAL Company Potential of Pharmaceutical Industry in Korea Aging populations and growing interest in health and welfare continue to drive up the demand for innovative drugs across the world. Globally, R&D spending in the pharmaceutical industry was higher than any other industry (pharmaceutical and bio ranked No. 1 with 150 billion dollars(2015)), indicating that the future industrial structure is shifting from IT to pharmaceutical business. Pharmaceutical Industry in Korea In Korean, the value of drugs manufactured in Korea was KRW 18.8 trillion in 2016. The annual average growth rate for the past five years was 4.6 percent. In addition, the size of the pharmaceutical market was KRW 21.7 trillion in 2016. Size and Market Trends in Pharmaceutical Industry in Korea (Unit: KRW Trillion) Category Production Export Import Balance Market size 2012 157,140 23,409 58,535 △35,126 192,266 2013 163,761 23,306 52,789 △29,483 193,244 2014 164,194 25,442 54,952 △29,510 193,704 2015 169,696 33,348 56,016 △22,668 192,364 2016 188,061 36,209 65,404 △29,195 217,256 YOY 10.8% 8.6% 16.8% - 12.9% CAGR(’12~’16) 4.6% 11.5% 2.8% - 3.1% Note: 1) Pharmaceutical products include finished products, narcotic drugs, ultra narcotic, psychotropic substance and drug substance 2) Numbers in export and import categories are calculated in Korean won and converted US dollar by using annual average exchange rate at Bank of Korea 3) Market Size= Production-Export+Import Source: Pharmaceutical Industry Statistics, Korea Pharmaceutical and Bio-Pharma Manufacturers Association Facts & Survey Report, Korea Pharmaceutical Traders Association, Korea Health Industry Statistics System Based on its technological competitiveness and quality drugs, the pharmaceutical industry in Korea has taken a stride in strengthening its ability to develop innovative drugs in a short period of time, including R&D, clinical trials and drug manufacturing. -

MINUTES of 229Th MEETING of CENTRAL LICENSING BOARD
MINUTES OF 257th MEETING OF CENTRAL LICENSING BOARD HELD ON JANUARY 24-25, 2018 *=*=*=*=* 257th meeting of the Central Licensing Board (CLB) was held on January 24-25, 2018 in the Committee Room, Drug Regulatory Authority of Pakistan, 4th Floor, T.F. Complex, G-9/4, Islamabad under the Chairmanship of Mr. Ghulam Rasool Dutani, Director Drug Licensing, Drug Regulatory Authority of Pakistan, Islamabad. Following members attended the meeting: - S. No. Name & Designation Status 1. Dr. Sheikh Akhter Hussain. Member Director (QA/LT), DRAP, Islamabad Mr. Munawar Hayat, Member 2. Chief Drug Controller, Primary and Secondary Health Care Department, Govt. of Punjab, Lahore Syed Welayat Shah, Member 3. Chief Drug Inspector, Department of Health, Govt. of Khyber Pakhtunkhwa, Peshawar Syed Saleem Shah, Member 4. Chief Drug Inspector, Department of Health, Govt. of Balochistan, Quetta 5. Syed Muied Ahmed, Expert in manufacturing of drugs. Member 6. Dr.Ikram-ul-Haque , Expert inQC/QA of drugs. Member 7. Prof. Dr. Abdullah Dayo, Dean, Faculty of Pharmacy, University of Member Sindh, Jamshoro 8. Prof. Dr. Mohammad Usman, Expert in manufacturing of drugs Member 9. Prof Dr. Jamshaid Ali Khan, Department of Pharmacy, University of Member Peshawar, Peshawar Mr. KhurramShahzad Mughal, Member 10. Consultant M/o Law, Justice and Human Rights, as representative of M/o Law and Justice, Islamabad. 11. Mr. Manzoor Ali Bozdar, Additional Director (Lic.), DRAP, Secretary/Member Islamabad. 12. Mr Sabooor Ahmed Sheikh and Mr. Arshad Mehmood as Observer Representatives of PPMA 13. Mr. Nadeem Alamgir, Representative of Pharma Bureau. Observer 14. Mr. Kamran Anwar, Representative of PCDA. Observer The meeting started with the recitation of verses from the Holy Qura’an. -

Sanofi Fires Viehbacher After Fall out with Board
OTC07-11-14p1_OTC15/11/2005 p1&24 05/11/2014 09:07 Page 1 7November 2014 COMPANY NEWS 3 Bayertakes leading spot 3 Sanofi fires Viehbacher in ChinawithDihon deal BoehringersaysnotoOmegabuy 3 GSKhints at Consumer divestments 4 afterfall out with board Merck waves goodbye to 5 Consumer Care business hris Viehbacher,chief executive officer Bayertargets critical mass 6 in Consumer Cof Sanofi, has been firedafter the firm’s board of directors concluded that his “man- Nasacortdrives salesforward 7 at Sanofi agement style was not adequate”. Oriola wantsafairprice 8 In asurprise move,the board of the French for itsRussian operations companyannounced it had “decided unani- BioGaiareports a 9 mously” to remove Viehbacher after six years double-digitsales rise at the helm. Celesioputsfaith in 11 “The group needs to pursue its development new leadership team with amanagement aligning the teams, harness- Pfizer expects Lipitortrialresults 12 ing talent and focusing on execution with aclose in 2015 and confident co-operation with the board,” Sanofistated, adding that its chairman, Serge GENERAL NEWS 13 Weinberg, would takecharge while it search- ed for achief executive. Most Germans have 13 Expanding on the rationale behind the deci- used homoeopathy sion, Weinbergsaid that the board was“strongly Chris Viehbacher has left Sanofi after the board Health Canada approves 16 dissatisfied” with Viehbacher’sability to work Aspirin heart-attack claim criticised his management style with board members to examine the company’s Germans reject OTCguidance 16 strategy “in aconfident manner”. (OTC bulletin,27February 2009, page 1). Australia settooverhaul 17 “The fluidity of the relationship between Acquisitions were central to Viehbacher’s TGAmedicines regulation the chief executive officer and the board was expansion strategy,with the companysnapping MARKETING NEWS 18 inadequate,”herevealed. -

Corporate Responsibility Report 2002
The Impact of Medicines Do more, feel better, live longer Corporate and Social Responsibility Report 2002 Corporate responsibility is an integral part of our business – it is inherent in the mission of our company. GlaxoSmithKline makes a significant positive contribution to society around the world, through the medicines and healthcare products that we research, develop, manufacture and sell. Contents 01 A global challenge 20 Valuing people 02 The scope of our business 24 Environment, health and safety 04 Our contribution to society 28 Business ethics and integrity 06 Medicines for the developing world 32 Management of CSR 12 Community investment 33 Web references 16 Research and development Impact of Medicines cover story Although a generation separates them, Bettina Bartels and her son Philippe are able to lead active lives despite both suffering from asthma. Their story can be found in our Annual Review. On page 5 of this report, Dr Vincent McGovern gives his perspective on asthma management as both a patient and a doctor. Annual Report Annual Review Corporate and Social Responsibility Report During 2002, we... • invested a total of £239 million in global community activities, product donations and charitable contributions – more than any other UK company; • donated 66 million albendazole tablets, worth £8.7 million, to 31 countries to support the prevention of lymphatic filariasis; • increased shipments of our Combivir HIV/AIDS treatment at preferential prices to the developing world to nearly 6 million tablets, up from 2.2 million in 2001; • worked with other companies to establish new industry Principles on the Conduct of Clinical Trials and Communication of Clinical Trial Results; and • surveyed 10,000 of our managers on their views of GSK’s Spirit and culture. -
General Pharmacopoeia Monograph
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Pharmaceutical forms GPM.1.4.1.0007.15 for parenteral administration Replaces the State Pharmacopoeia of the Russian Federation XI Monograph “Injectable pharmaceutical forms” The requirements of the present General Pharmacopoeia Monograph do not apply to immunobiological medicinal products, human blood-containing medicinal products, and radiopharmaceutical preparations designed for parenteral administra- tion. Homeopathic solutions for injection should additionally meet the require- ments of the General Pharmacopoeia Monograph “Homeopathic solutions for in- jection”. Pharmaceutical forms for parenteral administration are supplied as sterile dosage forms designed for administration to humans by means of injection, infu- sion, or implantation (involving impairment of the integrity of the skin or mucous membranes, not through the gastrointestinal tract). Pharmaceutical forms for parenteral administration include: – injectable and infusion pharmaceutical forms (solution for injection, emul- sion for injection, suspension for injection, solution for infusion, emulsion for infu- sion); – concentrates for injectable and infusion pharmaceutical forms; – solid pharmaceutical forms designed for preparation of injectable and infu- sion pharmaceutical forms (powder; lyophilized substance, including “freeze-dried powder”); – pharmaceutical forms for implantation (implant, tablet for implantation, etc.). Solution for injection (including “gel for injection”) – an aqueous or -
Evolution of Pharmacy
Evolution of Pharmacy INTRODUCTION: Dhanvantari: God of Ayurvedic Medicine & Physician of the Gods This was designed in order to reflect traditional culture of the land in the field of Pharmacy and Medicine. Dhanvantari, lord of Ayurvedic Healing, holds a golden leech (symbol of blood purification) and a medicinal plant in his right hand, and the Cronch of wisdom and Pot of rejuvenating nectar in his left . The tulsi-seed mala around his neck, plant-wreth halo, and his blue tinted skin emphasize his connection with Vishnu, the Preserver. Dhanvantari was an early Indian Practitioner and One of the World’s First Surgeons. He perfected many herbal based cures and natural remedies and was credited with the discovery of the Antiseptic Properties of Turmeric and Preservative Properties of Salt, which he incorporated in his cures. As a result of the Brilliance and achievements he displayed in the field of medicine, he was adjudged as One of the Nine Gems in early Indian ruler Vikramaditya’s court. PHARMACY DEFINITION: Pharmacy is the art and science of manufacturing and dispensing of drugs prepared by natural and synthetic sources, and using them for the treatment and prevention of diseases. PHARMACY SYMBOLS AND THEIR SIGNIFICANCE: A symbolic representation of the process of preparation of Medicine through crushing, grinding and mixing. Bowl of Hygieia is a greek Godess holding a Patera (Medicine Bowl) with a snake taxingly coiling. Symbolizing essential recipe in the form of Medicine Green cross symbolizes the sustained healthcare Learn → Transform → Elevate :: Bring Harmony & Peace Page 1 Evolution of Pharmacy HISTORY OF PHARMACY: Archeological evidence reveals that drug taking is an extremely old human phenomenon. -

|||||||III US005583122A United States Patent (19) 11) Patent Number: 5,583,122 Benedict Et Al
|||||||III US005583122A United States Patent (19) 11) Patent Number: 5,583,122 Benedict et al. 45) Date of Patent: Dec. 10, 1996 54) PHARMACEUTICAL COMPOSITIONS 100718 2/1984 European Pat. Off.. CONTAINING GEMINAL DIPHOSPHONATES 0170228 2/1986 European Pat. Off.. 2343476 4/1975 Germany. 75) Inventors: James J. Benedict, Norwich, N.Y.; 3. yals SNY shristopher M. Perkins, Cincinnati,- 53-59674 5/1978 Japan.ermany. O 54-135724 10/1979 Japan. (73) Assignee: The Procter & Gamble Company, 5.3 As E. Kingdom. Cincinnati, Ohio OTHER PUBLICATIONS 21 Appl. No.: 806,155 C.A. vol. 93(1980) 93: 232711f, Nissan Chem. Ind. (22 Filed: Dec. 6, 1985 C.A. vol.93(1980) 93: 199,239h, Nissan Chem. Ind. ( Worms Q.A. vol. 77 (1972) 130478N. Related U.S. Application Data C.A. vol. 94(1981) 94:11638f Nissan Chem. Ind. - - Francis & Martodam, "Chemical, Biochemical, & Medicinal (63) Contain-in-part of Ser. No. 684,543, Dec. 21, 1984, Properties of the Diphosphonates', in The Role of Phospho nates in Living Systems (CRC Press; Hilderbrand editor), pp. (51) Int. Cl. ..................... C07F 9/38; C07F 9/58; 55-96 (1983). A61K 31/675 Unterspann, "Experimental Examinations on the Suitability 52 U.S. C. .............................................. 514/89; 546/22 of Organoaminomethane-bis-Phosphonic Acids for 58) Field of Search ................................. 514/89; 546/23, Bone-Scintigraphy by Means of Tc-99m in Animals”, Eur. 546/22 J. Nucl. Med., vol. 1, pp. 151-154 (1976). Sologub et al., "Synthesis and Intramolecular Electronic (56) References Cited Interactions in Molecules of U.S. PATENT DOCUMENTS Tetrachloropyridyl-4-carbonimidoyl Dichloride and Its Derivatives", Khim. -

Dermatologic Pharmacology 1069
CHAPTER Dermatologic 61 Pharmacology Dirk B. Robertson, MD & Howard I. Maibach, MD CASE STUDY A 43-year-old woman presents with a complaint of worsen- papulopustular component of her acne rosacea. Recently, ing rosacea. She initially responded to once-daily topical she has noted increasing persistent facial erythema. What metronidazole 0.75% gel with excellent clearing of the therapeutic options are available? Diseases of the skin offer special opportunities to the clinician. In with short systemic half-lives. For example, once-daily applica- particular, the topical administration route is especially appropri- tion of corticosteroids appears to be just as effective as multiple ate for skin diseases, although some dermatologic diseases respond applications in many conditions. as well or better to drugs administered systemically. 4. Vehicles and occlusion: An appropriate vehicle maximizes the The general pharmacokinetic principles governing the use of ability of the drug to penetrate the outer layers of the skin. In drugs applied to the skin are the same as those involved in other addition, through their physical properties (moistening or dry- routes of administration (see Chapters 1 and 3). Although often ing effects), vehicles may themselves have important therapeu- depicted as a simple three-layered structure, human skin is a tic effects. Occlusion (application of a plastic wrap to hold the complex series of diffusion barriers (Figure 61–1). Quantitation drug and its vehicle in close contact with the skin) is extremely of the flux of drugs and drug vehicles through these barriers is the effective in maximizing efficacy. basis for pharmacokinetic analysis of dermatologic therapy, and techniques for making such measurements are rapidly increasing in number and sensitivity. -

Introduction of Kemiko Pharmaceuticals Ltd
Internship Report on The Recruitment & Selection Process and General HR Policies & Practices of Kemiko Pharmaceuticals Ltd. Internship Report on The Recruitment & Selection Process and General HR Policies & Practices of Kemiko Pharmaceuticals Ltd. Submitted to Mr. Tareq Mahbub Asst. Professor BRAC Business School BRAC University Prepared by Farzina Binty Masud ID# 11364017 Program : MBA BRAC Business School BRAC University Date: April 24, 2014 Letter of Transmittal April 24, 2014 Mr. Tareq Mahbub Assistant Professor BRAC Business School BRAC University, 66 Mohakhali C/A, Dhaka-1212. Subject : Submission of the Internship Report. Dear Sir, It is a great pleasure to submit my Internship Report on Kemiko Pharmaceuticals Ltd. (KPL), one of the leading pharmaceuticals company in Bangladesh. For preparing the report, I tried my best to relate and judge with all information from all available sources in the most realistic and efficient way. In this report, I have analyzed many aspects related to the HR Policies and Practices of the organization and emphasized on the Recruitment and Selection process. The knowledge and experience, which I have gathered, will surely help me in future. But there may be some mistakes due to various limitations. Therefore, I request your kind consideration in this regard. Any kind of suggestions and clarification will be accepted cordially. Sincerely Yours, Farzina Binty Masud Student ID # 11364017 Program: MBA i Acknowledgement The preparation of this report was almost impossible without the inspiration, sincere and utmost cooperation of the internship supervisor. So, I would like to express my indebtedness and deep sense of gratitude to my internship supervisor Mr. Tareq Mahbub (Asst.