Isolation and Characterization of Postsynaptic Densities from Various Brain Regions: Enrichment of Different Types of Postsynaptic Densities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

George Emil Palade Ia#I, Romania, 19 Nov
George Emil Palade Ia#i, Romania, 19 Nov. 1912 - Ia#i, Romania, 8 Oct. 2008 Nomination 2 Dec. 1975 Field Cell Biology Title Professor of Medicine in Residence, Emeritus, and Dean for Scientific Affairs, Emeritus, University of California, San Diego Commemoration – George Emil Palade was born on November 19, 1912, in Jassy, Romania. He studied medicine at the University of Bucharest, graduating in 1940. Already as a student, he became interested in microscopic anatomy and its relation to function and decided early to relinquish clinical medicine for research. After serving in the Romanian army during the Second World War, he moved to the United States in 1946, soon joining the laboratory of Albert Claude at the Rockefeller Institute for Medical Research, where, after Claude’s return to Belgium in 1949, he developed an independent laboratory, first in association with Keith Porter and later, after Porter’s departure in 1961, on his own. He stayed at what had become the Rockefeller University until 1973, when he moved to Yale University. His later years were spent at the University of California, San Diego, where he acted as Dean of Scientific Affairs. He passed away on 7 October 2008, after suffering major health problems, including macular degeneration leading to total blindness, a particularly painful ordeal for a man who had used his eyes all his life in a particularly creative way. He leaves two children from his first marriage with Irina Malaxa: Georgia Palade Van Duzen and Philip Palade. He married Marilyn G. Farquhar, a cell biologist, in 1971, after the death of his first wife. -

Philip Siekevitz 1918-2009
PHILIP SIEKEVITZ 1918-2009 A Biographical Memoir by DAVID D. SABATINI © 2012 National Academy of Sciences Any opinions expressed in this memoir are those of the author and do not necessarily reflect the views of the National Academy of Sciences. PHILIP SIEKEVITZ February 25, 1918–December 5, 2009 BY DAVID D. SABATINI 1 The text and references in this article originally appeared in the Journal of Cell Biology 189(2010):3-5, and are reprinted here with the journal’s permission. PHILIP SIEKEVITZ, AN EMERITUS PROFESSOR at the Rockefeller University who made pioneering contributions to the development of modern cell biology, passed away on December 5th, 2009. He was a creative and enthusiastic scientist, as well as a great experimentalist who throughout his lifetime transmitted the joy of practicing science and the happiness that comes with the acquisition of new knowledge. He was a man of great integrity, with a thoroughly engaging person- ality and a humility not often found in people of his talent. PHILIP SIEKEVITZ PHILIP Philip Siekevitz’s career proceeded along three phases marked by seminal warfare attacks. Because he was eager to enhance his scientific background, Siekevitz requested a transfer, contributions that opened up new avenues of research. The first phase was which resulted in his deployment as a laboratory techni- in the field of protein synthesis, in which he developed the first in vitro cian to an Air Force Supply Base for the Pacific War in system using defined cell fractions. Then, in collaboration with George San Bernardino, California, where he honed his skills in microscopy and chemical analysis. -
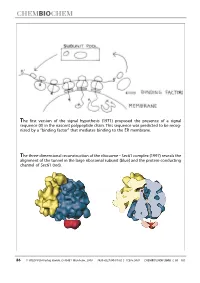
The First Version of the Signal Hypothesis (1971) Proposed the Presence of a Signal Sequence (X) in the Nascent Polypeptide Chain
The first version of the signal hypothesis (1971) proposed the presence of a signal sequence (X) in the nascent polypeptide chain. This sequence was predicted to be recog- nized by a ªbinding factorº that mediates binding to the ER membrane. The three-dimensional reconstruction of the ribosome ± Sec61 complex (1997) reveals the alignment of the tunnel in the large ribosomal subunit (blue) and the protein-conducting channel of Sec61 (red). 86 WILEY-VCH-Verlag GmbH, D-69451 Weinheim, 2000 1439-4227/00/01/02 $ 17.50+.50/0 CHEMBIOCHEM 2000,1,86±102 Protein Targeting (Nobel Lecture)** Günter Blobel*[a] KEYWORDS: membranes ´ Nobel lecture ´ proteins ´ protein transport Prologue I began research in the sixties, first as a graduate student in the laboratory of Van R. Potter (Figure 1) at the McArdle Institute for Cancer Research of the University of Wisconsin in Madison. I continued as a postdoctoral fellow in the laboratory of George E. Palade (Figure 2) at The Rockefeller Uni- versity in New York City. At that time, the intracellular pathway of secretory pro- teins, from their synthesis to their extru- sion from the cell, the so-called ªsecre- tory pathwayº, had already been worked out by George Palade and his co-workers, primarily Philip Siekevitz, Jim Jamieson, and Lucien Caro (for review see the 1974 Nobel lecture by George Palade[1] ). Using Figure 3. The secretory pathway. Secretory proteins (indicated in red) are pulse ± chase labeling with radioactive synthesized on ribosomes bound to the endoplasmic reticulum (ER). They are then amino acids in tissue slices in conjunction transported via vesicular carriers through the Golgi complex and are finally exocytosed. -

History of the Electron Microscope in Cell Biology
History of the Electron Advanced article Microscope in Cell Biology Article Contents . Introduction Barry R Masters, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA . Resolution and Its Limits in a Microscope . Early Development of the Electron Microscope . Transmission and Scanning Electron Microscopes In the years following World War II there was an explosion in the biological sciences with . Effect of the Electron Microscope in Neurobiology the rapid emergence of cell biology, molecular biology and biophysics. These events . Early Application of the Electron Microscope to Cell were affected by the development and use of new technologies for cellular fractionation Biology and imaging, specifically the electron microscope, which provided a resolution, that is, . Modern Applications of the Electron Microscope in unobtainable with light microscopes. Electron microscopes made visible the fine Biology structure of cells and their organelles, the structure of viruses. Now cryo-electron . Cryo-electron Microscopy microscopy is emerging as a key tool to visualize and localize the proteins in an entire . Concluding Remarks cell, the organization of actin filaments in the cytoskeleton, and molecular complexes . Glossary such as nuclear pores. Online posting date: 15th March 2009 Introduction enhancements are limited due to the physical principles of each type of microscope. One definition of resolution is the New developments in microscopic-imaging techniques ability to resolve two point sources of equal brightness (to aided -

Cell Nucleus, Mitochondrium, Peroxysome
Cell nucleus, mitochondrion, peroxysome Emese Pálfi Semmelweis University Department of Anatomy, Histology and Embryology - all organisms descended from a common ancestor cell - evolution by natural selection - cells are the smallest units of an organism - common features: cell membrane, DNA, cytoplasm - cell nucleus - cytoskeleton - RNA synthesis (transcription) and protein synthesis (translation) are separated The cell nucleus Nucelar envelope: Nuclear pores: - two concentric membane + - formed by nuclear pore complex perinuclear space - inner ring, annulus, outer ring - inner nuclear membrane is - selective active transport supported by the fetlike nuclear - nuclear localization signal (NLS) lamina (meshwork of interconnected - nuclear export signal (NES) proteins) - outer nuclear membrane is studded Nucleolus: with ribosomes - ribosomal RNA synthesis - directly connected to the - ribosome assembly endoplasmic reticulum (ER) Nucleoplasm - chromatin: DNA + histone proteins euchromatin: transcriptionally active less condensed heterochromatin: transcriptionally inactive highly condensed - non-histone proteins 5 µm - chromosomes are only visible during cell division - water, ions, RNA, soluble small molecules Barr-body: Inactive X-chromosome Chromosomes Somatic cells: two sets of 23 chromosomes 22 pairs of autosoms + 1 pair of sex chromosomes Mitochondrion established them as cell organelles Richard Altmann (1886) german pathologist mitos= "thread” chondrion= "granule" ”Powerhouse of the cell” Philip Siekevitz (1957) american biochemist -

Perspectives
PERSPECTIVES fractionation of liver homogenates. The TIMELINE emphasis was on the quantitative monitoring of the distribution of the chemical con- stituents of the cell, rather than organelle George Emil Palade: charismatic purity5–8. Trial and error must have been the norm, and cold-room stamina a prerequisite, virtuoso of cell biology but this early period ultimately established the procedures that allowed organelles to remain intact without agglutination or lysis. Many Alan M. Tartakoff obstacles confronted these investigators, including a “…biochemical Zeitgeist that par- George Palade has created, shared and on the state of knowledge at that time in his ticles were a nuisance and stood in the way passed on a multidisciplinary view of the Nobel lecture,“…biologists [had been] in the of purification of … enzymes.”9 Whereas functional organization, biogenesis and same situation as astronomers and astro- biochemistry was developing rapidly, the dynamics of organelles. His open- physicists, who were permitted to see the understanding of the compartmentalization mindedness and tenacity, along with his objects of their interest, but not to touch of subcellular activities and the significance of rigour and sense of intellectual elegance, them; the cell was as distant from us as the organelles was still in its infancy. have been remarkable. This focus on the stars and galaxies were from them. More dra- Claude returned to his native Belgium in logic of organelles defined a crucial turning matic and frustrating was that we knew that 1949, but not before he and his colleagues point in biomedical science. The following the instrument at our disposal, the micro- had systematized the use of differential sedi- article sketches Palade’s research, as part scope … had … reached, irremediably, the mentation to isolate a comprehensive set of of a larger community that flourished after theoretical limits of its resolving power.”4 fractions from tissue homogenates using the Second World War. -
![Dr. Christian De Duve] Fulvio Bardossi](https://docslib.b-cdn.net/cover/9000/dr-christian-de-duve-fulvio-bardossi-3979000.webp)
Dr. Christian De Duve] Fulvio Bardossi
Rockefeller University Digital Commons @ RU Rockefeller University Research Profiles Campus Publications Summer 1984 An Unconventional Traveler: [Dr. Christian de Duve] Fulvio Bardossi Judith N. Schwartz Follow this and additional works at: http://digitalcommons.rockefeller.edu/research_profiles Part of the Life Sciences Commons Recommended Citation Bardossi, Fulvio and Schwartz, Judith N., "An Unconventional Traveler: [Dr. Christian de Duve]" (1984). Rockefeller University Research Profiles. Book 21. http://digitalcommons.rockefeller.edu/research_profiles/21 This Article is brought to you for free and open access by the Campus Publications at Digital Commons @ RU. It has been accepted for inclusion in Rockefeller University Research Profiles by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. THE ROCKEFELLER UNIVERSITY Suddenly, right in front ofus, looms a dark, ungainly blimp...Col lision is inevitable .. .hurling us into what turns out to be a thoroughly unpleasant environment. Everywhere we look are scenes ofdestruction: maimed molecules ofvarious kinds, shapeless debris, hal/-recognizable pieces of bacteria and viruses, fragments of mitochondria, membrane RESEARCH whorls, damaged ribosomes, all in the process ofdissolving away before our very eyes. from "A Guided Tour of the Living Cell" CHRISTIAN DE DUVE, 1984 PROFILES SUMMER 1984 An Unconventional Traveler Christian de Duve In his Nobel LeCture, Chrisrian de Duve, Andrew W. Mellon Professor of The Rockefeller University, likened himself to a character in a tale who "traveled the whole of Sweden, from SHne to Lappland, on the wings ofa white gander. I, too," he said, "have made a wonderful journey, using, like Nils Hol gersson, an unconventional mode of travel." For thirty-five years Dr. -

Tweet About the Annual Meeting: Use #ASBMB2010
TweeT abouT The annual meeTing: use #ASBMB2010 April 2010 American Society for Biochemistry and Molecular Biology Abstract Submission and Registration Opening Soon! September 30 – October 4, 2010 Transcriptional Regulation by Chromatin and RNA Polymerase II Granlibakken Resort, Tahoe City, CA Organizer: Ali Shilatifard Stowers Institute for Medical Research October 14 – October 17, 2010 Biochemistry and Cell Biology Of ESCRTs in Health and Disease Snowbird Resort, Snowbird, UT Organizer: James Hurley National Institute of Diabetes and Digestive and Kidney Diseases Phyllis Hanson Washington University School of Medicine October 21 – October 24, 2010 Detection and Physiological Evaluation Granlibakken Resort, Tahoe City, CA Organizer: Katalin Medzihradszky University of California, San Francisco Gerald Hart Johns Hopkins University School of Medicine October 28 – October 31, 2010 Secretory and Endocytic Pathways Granlibakken Resort, Tahoe City, CA Organizer: Stanford University School of Medicine Vivek Malhotra Center for Genomic Regulation, Barcelona, Spain www.asbmb.org/meetings contents April 2010 On the cover: Read about the work of Jorge Cham, society news our cover artist, 2 Letters to the Editor and other science cartoons. 16 4 President’s Message Cover imAge reprinted with permission from Jorge ChAm. 6 News from the Hill 9 Washington Update 10 ASBMB Journal Innovations 11 ASBMB Announces Diversity in Science Award 12 Retrospective: Philip Siekevitz (1918 – 2009) Social Media at the Annual Meeting. 13 Retrospective: 23 Marshall Nirenberg (1927 – 2010) 14 Member Spotlight feature stories 16 The Graduate Student Experience, In Four Panels 2010 annual meeting 20 Getting the Most Out of the Annual Meeting 22 Back by Popular Demand: The Annual Meeting Compendia in every issue 23 Sci.comm The ASBMB 2010 special symposia lineup. -

Identification of Homo-Oligomers As Potential Intermediates In
Proc. Natt Acad. Sci. USA Vol. 80, pp. 4359-4363, July 1983 Cell Biology Identification of homo-oligomers as potential intermediates in acetylcholine receptor subunit assembly (multi-subunit membrane protein/detergent solubilization/sucrose gradient analysis/homologous associations/early biosynthetic events) DAVID J. ANDERSON* AND GUNTER BLOBEL Laboratory of Cell Biology, The Rockefeller University, New York, New York 10021 Communicated by Philip Siekevitz, April 4, 1983 ABSTRACT We have examined the sedimentation behavior, sucrose density gradients. The subunits each appear to self-as- on sucrose density gradients, of acetylcholine receptor (AcChoR) sociate, forming homo-oligomers in the range of 7-13 S. Such subunits synthesized in vitro and integrated into heterologous rough homologous associations were not observed for another mem- microsomal membranes. In media containing nondenaturing de- brane protein, opsin, studied in an identical system in parallel. tergents such as Triton X-100 or deoxycholate, the subunits ap- These AcChoR homo-oligomers are suggested to function in the pear to self-associate although, as previously reported, no het- stabilization of hydrophilic surfaces that will eventually border erologous interactions were detected. The sedimentation profiles the ion channel in the mature complex, during the lengthy pe- assume a broad distribution in the region of 7-13 S. However, the riod of transport to the site of assembly of the functional re- peak fractions occupy the same region of the gradient as does na- ceptor. tive AcChoR, run in parallel. Such large homo-oligomers were not observed for another membrane protein, opsin, studied in the same way. This indicated that the associations are indeed between the MATERIALS AND METHODS AcChoR subunits and not simply between all newly synthesized membrane proteins. -

The Rockefeller Institute Quarterly 1958, Vol. 2, No. 2 the Rockefeller University
Rockefeller University Digital Commons @ RU The Rockefeller Institute Quarterly The Rockefeller University Newsletters Summer 1958 The Rockefeller Institute Quarterly 1958, vol. 2, no. 2 The Rockefeller University Follow this and additional works at: http://digitalcommons.rockefeller.edu/ rockefeller_institute_quarterly Recommended Citation The Rockefeller University, "The Rockefeller Institute Quarterly 1958, vol. 2, no. 2" (1958). The Rockefeller Institute Quarterly. Book 6. http://digitalcommons.rockefeller.edu/rockefeller_institute_quarterly/6 This Book is brought to you for free and open access by the The Rockefeller University Newsletters at Digital Commons @ RU. It has been accepted for inclusion in The Rockefeller Institute Quarterly by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. VOLUME 2 NUMBER 2 SUMMER 1958 BIOCHEMICAL GENETICS AND The life cycle of Neurospora was well understood many years ago, thanks to the studies of Dr. B. 0.Dodge at the New INHERITED METABOLIC DISEASE York Botanical Garden. It is a microorgan- ism that exists in two sexes so that cross- THE IDEA THAT not only gross traits of which cause them are in some cases rather breeding experiments are possible, and the species and individuals are genetically de- well understood. Wilson's disease, which cycle from infancy to maturity is only lo termined but that even detailed biochem- we shall describe later, is one of them. But days. The fungus also multiplies asexually, ical processes are gene-controlled owes on the whole, man is not a very suitable that is by simple division, so that billions much to the work of Dr. Edward L. Tatum, organism for any kind of genetic studies. -

The Rockefeller Institute Quarterly 1958, Vol.2, No.1 the Rockefeller University
Rockefeller University Digital Commons @ RU The Rockefeller Institute Quarterly The Rockefeller University Newsletters Spring 1958 The Rockefeller Institute Quarterly 1958, vol.2, no.1 The Rockefeller University Follow this and additional works at: http://digitalcommons.rockefeller.edu/ rockefeller_institute_quarterly Recommended Citation The Rockefeller University, "The Rockefeller Institute Quarterly 1958, vol.2, no.1" (1958). The Rockefeller Institute Quarterly. Book 5. http://digitalcommons.rockefeller.edu/rockefeller_institute_quarterly/5 This Book is brought to you for free and open access by the The Rockefeller University Newsletters at Digital Commons @ RU. It has been accepted for inclusion in The Rockefeller Institute Quarterly by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. VOLUME 2 NUMBER 1 BACTERIOLOGY, GENETICS, AND DNA- Strangely enough, a bacteriologist at the Rockefeller Institute who was absorbed in studying pneumonia made the most signi- A FRONTIER IN RESEARCH ficant step in discovering the key role of DNA in the regulation of living organisms. MOST BIOCHEMISTS and geneticists in the case of the human ovum, for ex- The late Dr. 0. T. Avery found that DNA today agree that deoxyribonucleic acid, or ample) contain all the information neces- extracted from the cells of one strain of DNA,is an essential part of the mechanism sary to enable it to elaborate itself into a pneumococci can transform another strain of heredity. Outstanding contributions to mature organism capable of carrying on into the first. But most important-the our understanding of the importance of the species. A central problem in genetics change is passed from generation to gen- DNA have been made by scientists of the research today is the attempt to discover eration. -

Recent Developments in Mitochondrial Medicine (Part 1)
4open 2021, 4,2 Ó V. Weissig & M. Edeas, Published by EDP Sciences, 2021 https://doi.org/10.1051/fopen/2021002 Available online at: www.4open-sciences.org REVIEW ARTICLE Recent developments in mitochondrial medicine (Part 1) Volkmar Weissig1,* and Marvin Edeas2,3 1 Midwestern University, College of Pharmacy, Department of Pharmaceutical Sciences, 19555 N. 59th Avenue, Glendale, AZ 85308, USA 2 Université de Paris, INSERM U1016, Institut Cochin, CNRS UMR8104, Faculté de médecine Cochin-Port Royal, 75014 Paris, France 3 Laboratory of Excellence GR-Ex, 75015 Paris, France Received 15 February 2021, Accepted 15 April 2021 Abstract – Research into elucidating structure and function of mitochondria has been quite steady between the time of discovery during the end of the 19th century until towards the late 1980’s. During the 1990s there was talk about a “comeback” of this organelle reflecting a widely revitalized interest into mitochondrial research which was based on two major discoveries made during that time. The first was the etiological association between human diseases and mitochondrial DNA mutations, while the second revealed the crucial function of mitochondria during apoptosis. The March 5th, 1999 issue of Science even featured a textbook image of a mitochondrion on its front cover and was entirely dedicated to this organelle. Whilst the term “comeback” might have been appropriate to describe the general excitement surrounding the new mitochondrial discoveries made during the 1990s, a term for describing the progress made in mitochondrial research during the last two decades is difficult to find. Between 2000 and 2020 the number of publications on mitochondria has skyrock- eted.