Tasmania's Genetically Modified Organisms (GMO) Moratorium
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
03 03 2015 (Pdf)
The NAWG officers were joined by Sen. Pat Roberts, chairman of the Senate Ag Spending more time with his family is one of his priorities as Penner retires from his Committee, following a productive discussion about issues important to the wheat in- position as NAWG president. Courtesy photos dustry. Made from scratch: outgoing NAWG president Paul Penner recalls his work on wheat By Julia Debes eration is mostly no-till and actively involved in the ef- consuming, but the lessons their livelihoods, they were short of their own ag educa- Kansas wheat farmer Paul includes wheat, corn, soy- fort to create Heartland Plant learned from working with able to get the legislation tion. Penner is equally likely to beans and hay, in addition to Innovations, the for-profit wheat farmers in 22 states is passed. “They are not going to be share updates from Capitol an occasional rotation of company developed by worth it. “It was a big struggle to naïve about where their food Hill or pictures of his grand- sorghum. Kansas wheat farmers to “It is amazing the amount get that through,” he said. “It comes from. I want to instill kids. While Penner will soon Penner started attending provide advanced plant of knowledge you can ac- really brought a lot of farm- all of this love of agriculture retire as president of the Na- local Kansas Association of breeding services for wheat quire if you are observant,” ers together.” and food in them. I hope it is tional Association of Wheat Wheat Growers (KAWG) and other crops, now spe- he said. -

The Lure of Distant Shores Income Is Derived from Any Public Assistance Program
l a r u R USDA / RurCCal DevelOOopment OOPPEE RRAATTIIVVEEMay/JSuS ne 2013 The Lure of Distant Shores Co-ops share export strategies Commentary The Co-op Way By Dallas Tonsager, Former Under Secretary Sometimes the enthusiasm that helps launch a co-op can USDA Rural Development dissipate over time as it passes from one generation to the next. While new co-ops can learn much from older, grew up in the “co-op culture” of rural established co-ops, the latter can also benefit from the sense I South Dakota, relying on co-ops for of excitement and “all for one” that we see among the new everything from farm supplies to electricity. crop of co-ops. So I’ve always had a healthy respect for how Co-ops have never been afraid of a challenge. Indeed, vital co-ops are to rural America. But the past many, if not most, of our successful co-ops were created in 4 ½ years serving as Under Secretary for USDA Rural challenging times. Thousands were formed in the 1930s- Development has given me an even greater appreciation of 1960s, fueled by an absolute need — such as the need for a how these user-owned, user-controlled businesses are helping source of quality farm supplies and electricity at affordable to build a stronger rural economy. prices, or the need to market and add value to crops in order I have had the good fortune to spend 17 of the past 20 to gain clout in the marketplace. years working in the federal government. -

2020 World Championship Cheese Contest
2020 World Championship Cheese Contest Winners, Scores, Highlights March 3-5, 2020 | Madison, Wisconsin ® presented by the Cheese Reporter and the Wisconsin Cheese Makers Association World Cheese Contest ® Champions 2020 1998 1976 MICHAEL SPYCHER & PER OLESEN RYKELE SYTSEMA GOURMINO AG Denmark Netherlands Switzerland 1996 1974 2018 HANS DEKKERS GLEN WARD MICHEL TOUYAROU & Netherlands Wisconsin, USA SAVENCIA CHEESE USA France 1994 1972 JENS JENSEN DOMENICO ROCCA 2016 Denmark Italy TEAM EMMI ROTH USA Fitchburg, Wisconsin USA 1992 1970 OLE BRANDER LARRY HARMS 2014 Denmark Iowa, USA GERARD SINNESBERGER Gams, Switzerland 1990 1968 JOSEF SCHROLL HARVEY SCHNEIDER 2012 Austria Wisconsin, USA TEAM STEENDEREN Wolvega, Netherlands 1988 1966 DALE OLSON LOUIS BIDDLE 2010 Wisconsin, USA Wisconsin, USA CEDRIC VUILLE Switzerland 1986 1964 REJEAN GALIPEAU IRVING CUTT 2008 Ontario, Canada Ontario, Canada MICHAEL SPYCHER Switzerland 1984 1962 ROLAND TESS VINCENT THOMPSON 2006 Wisconsin, USA Wisconsin, USA CHRISTIAN WUTHRICH Switzerland 1982 1960 JULIE HOOK CARL HUBER 2004 Wisconsin, USA Wisconsin, USA MEINT SCHEENSTRA Netherlands 1980 1958 LEIF OLESEN RONALD E. JOHNSON 2002 Denmark Wisconsin, USA CRAIG SCENEY Australia 1978 1957 FRANZ HABERLANDER JOHN C. REDISKE 2000 Austria Wisconsin, USA KEVIN WALSH Tasmania, Australia Discovering the Winning World’s Best Dairy Results Wisconsin Cheese Makers Association was honored to host an international team of judges and an impressive array of samples of 2020 cheese, butter, yogurt and dairy ingredients from around the globe at the 2020 World Championship Cheese Contest March 3-5 in Madison. World Champion It was our largest event ever, with a breath-taking 3,667 entries from Michael Spycher, Mountain 26 nations and 36 American states. -
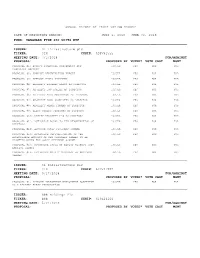
Annual Report of Proxy Voting Record Date Of
ANNUAL REPORT OF PROXY VOTING RECORD DATE OF REPORTING PERIOD: JULY 1, 2018 - JUNE 30, 2019 FUND: VANGUARD FTSE 250 UCITS ETF --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ISSUER: 3i Infrastructure plc TICKER: 3IN CUSIP: ADPV41555 MEETING DATE: 7/5/2018 FOR/AGAINST PROPOSAL: PROPOSED BY VOTED? VOTE CAST MGMT PROPOSAL #1: ACCEPT FINANCIAL STATEMENTS AND ISSUER YES FOR FOR STATUTORY REPORTS PROPOSAL #2: APPROVE REMUNERATION REPORT ISSUER YES FOR FOR PROPOSAL #3: APPROVE FINAL DIVIDEND ISSUER YES FOR FOR PROPOSAL #4: RE-ELECT RICHARD LAING AS DIRECTOR ISSUER YES FOR FOR PROPOSAL #5: RE-ELECT IAN LOBLEY AS DIRECTOR ISSUER YES FOR FOR PROPOSAL #6: RE-ELECT PAUL MASTERTON AS DIRECTOR ISSUER YES FOR FOR PROPOSAL #7: RE-ELECT DOUG BANNISTER AS DIRECTOR ISSUER YES FOR FOR PROPOSAL #8: RE-ELECT WENDY DORMAN AS DIRECTOR ISSUER YES FOR FOR PROPOSAL #9: ELECT ROBERT JENNINGS AS DIRECTOR ISSUER YES FOR FOR PROPOSAL #10: RATIFY DELOITTE LLP AS AUDITORS ISSUER YES FOR FOR PROPOSAL #11: AUTHORISE BOARD TO FIX REMUNERATION OF ISSUER YES FOR FOR AUDITORS PROPOSAL #12: APPROVE SCRIP DIVIDEND SCHEME ISSUER YES FOR FOR PROPOSAL #13: AUTHORISE CAPITALISATION OF THE ISSUER YES FOR FOR APPROPRIATE AMOUNTS OF NEW ORDINARY SHARES TO BE ALLOTTED UNDER THE SCRIP DIVIDEND SCHEME PROPOSAL #14: AUTHORISE ISSUE OF EQUITY WITHOUT PRE- ISSUER YES FOR FOR EMPTIVE RIGHTS PROPOSAL #15: AUTHORISE MARKET PURCHASE OF ORDINARY ISSUER YES FOR FOR -

A Golden Future for Corn? Riparian Areas
A FLOWERING BULLDoZERS BUSINESs bANNED Carolyn Bondy got into daylilies Rancher turns down $1 million, donates by accident, and now sells them land for a provincial park » PG 32 across the country » PG 3 Publications Mail Agreement # 40069240 Volume 12, number 17 A u g ust 17, 2015 Doing the right thing — A FRIEND IN HARD TIMES and getting paid for it ALUS program shares cost of stewardship projects with farmers By JENNIFER BLAIR AF stAFF / sylvAn lAke ike most farmers, kevin Ziola wants to be a good L steward of the land — but it’s been tough for the third- generation farmer to balance his conservation efforts with his bot- tom line. “As a cattle farmer, I believe it’s important to work with nature, not against it,” said Ziola, who runs 200 head of cattle on 10 quarters near sylvan lake with wife Roxanne. “But we don’t make lots of money, so it’s hard to put away a little extra cash for (conserva- When drought sent hay prices soaring, Cindy Wilinski harnessed the power of social media to get affordable hay tion). It wouldn’t be that high on to those who need it most. Read the full story on Page 12. PHOTO: WENDY DUDLEY the list because machinery, cattle, and feed take priority.” But thanks to a national pro- gram called AlUs (pronounced ‘Alice’ and short for Alternative land Use services), farmers like the Ziolas can now get paid to retain or reconstruct natural areas such as wetlands, grasslands, and A golden future for corn? riparian areas. -

Inside Taiwan Gets Fresh VOL
AUGUST 2020 DAIRYDAIRY INDUSTRIES international INDUSTRIES www.dairyindustries.com AUGUST 2020 DAIRY INDUSTRIES INTERNATIONAL DAIRY Bubble tea travels Inside Taiwan gets fresh VOL 85 No 8 Focus on filtration Environmental aspects Cellular level milk Dii COVER AUG 20**.indd 1 15/07/2020 10:12 Contents REGULARS AUGUST 2020 DAIRY DAIRY international international INDUSTRIES INDUSTRIES INDUSTRIES 5 Editor’s Comment www.dairyindustries.com AUGUST 2020 Volume 85 No. 8 6 World News DAIRY INDUSTRIES INTERNATIONAL DAIRY August 2020 14 New Product News 36 New Equipment 37 Diary Bubble tea Editorial Director 42 Working Day... Ross McMahon, CEO, Kendal Nutricare travels Sarah McRitchie Inside [email protected] Taiwan gets fresh FEATURES 85 No 8 VOL Focus on filtration Environmental aspects Editor Cellular level milk 12 News Focus Suzanne Christiansen Dii COVER AUG 20**.indd 1 15/07/2020 10:12 [email protected] Capitalising on consumer demands for functional products News Focus Art Editor 13 Sue Burke US export performance was greater than expected [email protected] 16 Euro View Digital Editor The EU dairy sector is fighting through Covid-19, but Alex Rivers political storms are threatening the industry’s health [email protected] 18 Environment Regular Contributors The Innovation Center for US Dairy has selected six Henrik von Suhr winners for its sustainability awards this year Julian Mellentin Environment 21 Dairy Beverages See page 18 Group Sales Manager A RTD bubble tea debuts for grab and go convenience Mark -

1 Katrina Navickas What Happened to Class? New Histories of Labour and Collective Action in Britain. Recently, My Family Called
1 Katrina Navickas What Happened to Class? New Histories of Labour and Collective Action in Britain. Recently, my family called me a „labour historian‟. A „labour historian‟ is one of the last epithets I would give to my thoroughly bourgeois self, so I considered why they made that association. In 2005, I published an article rethinking Luddism, the machine– breaking outbreaks of 1812. It stuck out somewhat incongruously as an old–fashioned topic, although I had reworked it with a postmodernist nod towards the agency of language.1 In the heyday of labour history in the 1960s and 1970s, it was a natural assumption to connect the study of trade unions and the Labour party with labour‟s more troublesome sister, social movements and popular protest. Yet over the past couple of decades, labour history has changed. Many of its historians no longer regard the labour (and Labour) movement as the be–all and end–all of the history of the working class. Their interests have diversified, shedding new light on identities and activities that are not completely subsumed by a narrative of class. Perhaps, indeed, I had mistaken myself for a labour historian of the old sort, even though methodologically and culturally I was far from being so. Although I did not realise it at the time, however, protest history had begun to be rethought and revived in a new direction. This is a review of recent developments in British labour and collective action history. In 2009, I returned to mythical leaders of machine–breakers. This time they were in the form of „Captain Swing‟, that head of the eponymous rural agitation of the early 1830s. -

The Revolt of the Fields in East Anglia 4/- 50 (Alf Peacock)
I N M E M O R I A who has been Secretary of the History Group of the Communist Party for several years, died on Tuesday, 19th April. Without his lively interest and constant practical help OUR HISTORY would not have appeared, nor would the other activities of the History Group have taken place. He was typical of the best in the British working-class, a lifelong Communist, educated by the Communist Party and by his own wide experiences and self-study. In recent years he had been responsible for many developments in the educational and cultural work of the Party. His vast knowledge of people and his deep humanism, was always at the service of professional and specialist groups. His modesty was an example to all who worked with him; it cloaked but did not conceal from the perceptive a penetrating mind and considerable learning. We shall miss him greatly,: His monument is in the developing influence of Marxist ideas in many varied aspects of our culture. Page One FOREWORD Mr. Peacock's "Bread or Blood" (Gollancz, 1965) did not receive the attention which it deserved. We suspect because its combination of massive documentation in the local press and local records with unwavering sympathy for the cause of the exploited and oppressed made the academic and reviewing establishment uneasy. Mr. Peacock has now turned his attention from the 'agrarian riots in East Anglia in 1816' to 'the agricultural trade unionism of the 1870s' in the same area. (l) We are pleased to be able to publish this study, although Mr. -

2019 Results ICDA.Pdf
Class Description Gold Silver Bronze VHC VHC DP1 Best Showdressed Farmhouse / Joseph Heler Ltd 4 Traditional Cheese in the following classes DP2 to DP19. Automatic Entry Showdressed cheese DP2 Farmhouse / Traditional Cheshire Carron Lodge 10 Belton Farm Limited1 Belton Farm Limited 5 Cheese - White. Any weight. Traditional Cheshire Cheese Trad White Cheshire Trad White Cheshire DP3 Farmhouse / Traditional Cheshire Belton Farm Limited 3 Applebys 4 Belton Farm Limited 1 Dewlay 2 Cheese - Coloured. Any weight. Cheesemakers Trad Coloured Cheshire Raw Milk Traditional Trad Coloured Cheshire Coloured Cheshire DP4 Farmhouse / Traditional Mild Belton Farm Limited 1 Hayfields Dairy 4 Hayfields Dairy 2 Cheddar - White or Coloured. Any weight Mild Coloured Cheddar - Top Hat DP5 Farmhouse / Traditional Medium Hayfields Dairy 1 Hayfields Dairy 3 Barbers Farmhouse 2 Cheddar - White or Coloured. Any Cheesemakers weight Farmhouse Medium Cheddar Sponsored by : - High quality ingredients for the dairy industry 1 Class Description Gold Silver Bronze VHC VHC DP6 Farmhouse / Traditional Mature Trethowans Dairy 8 Keens Cheddar Raw 7 Ashley Chase Estate 1 Barbers Farmhouse 3 Cheddar - White or Coloured. Any Ltd Milk Cheddar Cheesemakers weight Pitchfork - organic, Raw milk Traditional Cave aged cheddar Farmhouse Mature Cheddar unpasteurised cheddar Mature Cheddar DP7 Farmhouse / Traditional Extra Ashley Chase Estate 4 Barbers Farmhouse 5 Keens Cheddar Raw 3 Matured Cheddar - White or Cheesemakers Milk Cheddar Coloured. Any weight Cave aged cheddar Farmhouse Extra Mature Raw Milk Traditional Extra Cheddar Mature Cheddar DP8 Farmhouse / Traditional Vintage Ashley Chase Estate 1 Barbers Farmhouse 5 Butlers Farmhouse 6 Cheddar - White or Coloured. Any Cheesemakers Cheeses weight Cave aged cheddar Farmhouse Vintage Cheddar Farmhouse Vintage Cheddar DP9 Farmhouse / Traditional Crumbly Greenfields dairy 3 Greenfields dairy 9 Dewlay 8 Lancashire Cheese. -

1767B-1 Rd-Gd-2008-56
Disclaimer: The contents of this guidance document does not have the force and effect of law and is not meant to bind the public in any way. This document is intended only to provide clarity to the public regarding existing requirements under the law or agency policies. UNITED STATES DEPARTMENT OF AGRICULTURE Rural Utilities Service BULLETIN 1767B-1 RD-GD-2008-56 SUBJECT: · Uniform System of Accounts - Electric TO: All Electric Borrowers RUS Electric Staff ProgramAccounting Services Division EFFECTIVE DATE: May 27, 2008 EXPIRATIOND ATE: Date of change in 7 CPR Part 1767 byrulemaking OFFICE OF PRIMARY INTEREST: Technical Accounting and Auditing Staff, Program Accounting Services Division PREVIOUS PUBLICATIONS: This bulletin replaces RUS Bulletin 1767B-1, Uniform System of Accounts - Electric, dated Sept ember 1, 1997. FILING INSTRUCTIONS: Discard RUS Bulletin 1767B-1, UniformS ystem of Accounts - Electric, dated September 1, 1997. PURPOSE: This bulletin sets forth, in a more user-friendly format, the RUS Uniform System of Accounts (USoA) and accounting interpretationsfor electric borrowers. This bulletin is a reprint of already codifiedpo licies and procedures found in 7 CPR Part 1767, Accounting Requirements for RUS Electric Borrowers, revised as of May 27, 2008. This bulletin is for use by borrowers, consultants, and other interested parties. Every effort has been made to ensure the accuracy of this document. However, in case of discrepancies, the regulations at 7 CPR Part 1767 are the authorized sources. SEP 1 7 ZUO� Date Bulletin 1767B-1 -

The Wyoming Rural Development Council
The Wyoming Rural Development Council The Wyoming Rural Development Council is a collaborative public/private partnership that brings together six partner groups: local/regional government, state government, federal government, tribal government, non-profit organizations, and private sector individuals and organizations. WRDC is governed by a Board of Directors representing the six partner groups. The Board as well as the Council membership have established the following goals for the WRDC: Assist rural communities in visioning and strategic planning Serve as a resource for assisting communities in finding and obtaining grants for rural projects Serve and be recognized as a neutral forum for identification and resolution of multi- jurisdictional issues. Promote, through education, the understanding of the needs, values and contributions of rural communities. The Council seeks to assist rural Wyoming communities with their needs and development efforts by matching the technical and financial resources of federal, state and local governments and the private sector with local development efforts. If you would like more information about the Wyoming Rural Development Council and how you may benefit as a member, contact: Mary Randolph, Executive Director Wyoming Rural Development Council 214 W. 15th Street Cheyenne, WY 82002 307-777-6430 307-777-2935 (fax) [email protected] www.wyomingrural.org Table of Contents Town of Encampment Five-Year Follow Up Resource Team Community Assessment Report September 20 & 21, 2010 Process for the Development of the Team Study and Report…………………….. 3 Executive Review………………………………………………………………. 4 Encampment Community Profile…………………………………………… …… 5 Resource Team Members and Community Planning Team……………………… 12 Agenda…………..…………………………………………………………….….. 13 Major Themes…………………………………………………………………….. 14 Recommendations Submitted…………………………………………………….. 15 By Team Members Derrel Carruth Jo Ferguson Nancy Olson Kathy Wilder What Was Said In the Interviews………………………………………………… 47 20 Clues to Rural Community Survival.…………………………………………. -

Inspiring Innovation Across the Agrifood Sector
Inspiring Innovation across the agrifood sector Who we are The UK Agri-Tech Centres are a unique collaboration between Government, academia and industry to drive greater efficiency, resilience and wealth across the agrifood sector. • Joining-up existing research excellence • Investing in new innovative resources and research • Addressing challenges that no one part of the agrifood sector can address alone • Positioning the UK as a global leader in sustainable food production Who we work with Together, the four Centres serve the needs of everyone involved in the UK agrifood sector: from farmers to advisors and the supply trade pre-farmgate; scientists to suppliers; processors to retailers. Our reach extends across all key industry players, trade associations and government. We are a shared voice to inform and influence industry priorities and ensure important industry issues are addressed. Why work with us The Centres are essential catalysts for change. We provide a gateway for companies and individuals seeking access to the very best science, expertise and technologies - stimulating new research, practice and technology for the agrifood sector. We are also building on existing knowledge, stimulating new research and technology as well as the transfer of knowledge across the whole sector. Each Centre has its own unique focus; offering capabilities that can lead the world in delivering sustainable food and farming solutions. Agri-EPI CHAP Engineering, precision & innovation for sustainable food Advancing crop productivity for future generations. production. CHAP is a catalyst for change, bringing together researchers, Agri-EPI supports and delivers research, development, industry and government to accelerate the identification, demonstration and training on precision agriculture and development and adoption of innovative agri-tech crop solutions engineering to maximise the agri‐tech sector’s contribution to to transform UK and global farming systems sustainably.