Cesium and Strontium Incorporation Into Zeolite-Type Phases During Homogeneous Nucleation from Caustic Solutions
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter
catalysts Article Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter Volker Mauer 1,* , Christian Bläker 1, Christoph Pasel 1 and Dieter Bathen 1,2 1 Chair of Thermal Process Engineering, University of Duisburg-Essen, Lotharstraße 1, 47057 Duisburg, Germany; [email protected] (C.B.); [email protected] (C.P.); [email protected] (D.B.) 2 Institute of Energy and Environmental Technology e.V. (IUTA), Bliersheimer Straße 60, 47229 Duisburg, Germany * Correspondence: [email protected]; Tel.: +49-203-3792176 Abstract: In addition to the adsorption mechanism, the heat released during exothermic adsorption influences the chemical reactions that follow during heterogeneous catalysis. Both steps depend on the structure and surface chemistry of the catalyst. An example of a typical catalyst is the faujasite zeolite. For faujasite zeolites, the influence of the Si/Al ratio and the number of Na+ and Ca2+ cations on the heat of adsorption was therefore investigated in a systematic study. A comparison between a NaX (Sodium type X faujasite) and a NaY (Sodium type Y faujasite) zeolite reveals that a higher Si/Al ratio and therefore a smaller number of the cations in faujasite zeolites leads to lower loadings and heats. The exchange of Na+ cations for Ca2+ cations also has an influence on the adsorption process. Loadings and heats first decrease slightly at a low degree of exchange and increase significantly with higher calcium contents. If stronger interactions are required for heterogeneous catalysis, then the CaNaX zeolites must have a degree of exchange above 53%. The energetic contributions show that the highest-quality adsorption sites III and III’ make a contribution to the load-dependent heat of adsorption, which is about 1.4 times (site III) and about 1.8 times (site III’) larger than that of adsorption site II. -

Preparation of Synthetic Zeolites from Myanmar Clay Mineral Technical
MM0800096 Preparation of Synthetic Zeolites From Myanmar Clay Mineral Technical Report (No. 1017/1/2004) By Dr. Phyu Phyu Win Ceramics Research Department Myanma Scientific and Technological Research Department (April, 2004) ABSTRACT Faujasite type zeolite X was successfully synthesized from Myanmar clay mineral, kaolinite. by treating with sodium hydroxide at 820 C followed by dissolution in water and hydrothermal treatment. It was found that the solution of fused clay powder can be crystallized at 90 °C under ambient pressure to synthesize faujasite type zeolite X. The effects of aging time and the amount of water on the formation of the product phase and Si/ AI ratios of the resulting products were investigated. Most of the Si and Al components in kaolinite might be dissolved into an alkaline solution and reacted to form ring-like structures. Then it was effectively transformed into zeolite materials. The maximum relative crystallinity of faujasite zeolite obtained was found to be 100%. Zeolite P was found to be a competitive phase present in some resulting products during hydrothermal treatment. The cation exchange capacity of kaolinite is very low, but increased after a proper treatment. It was found that the prepared faujasite type zeolite X, zeolite P and hydrogen zeolite (HZ) can reduce the hardness, the alkalinity, the total dissolved solid and the dissolved iron of raw water in the batch wise operation of water treatment. Therefore, it can be used as the cation exchanged resin for water treatment. Key words: kaolinite. zeolite P7 faujasite, zeolite X. ACKNOWLEDGMENT Financial support from the Myanma Scientific and Technological Research Department is gratefully acknowledged. -

Single-Crystal Structure of Fully Dehydrated Sodium Zeolite Y (FAU)
ANALYTICAL SCIENCES 2006, VOL. 22 x209 2006 © The Japan Society for Analytical Chemistry X-ray Structure Analysis Online Single-crystal Structure of Fully Dehydrated Sodium Zeolite Y (FAU), Na71[Si121Al71O384]-FAU Sung Man SEO,*1 Ghyung Hwa KIM,*2 Heung Soo LEE,*2 Seong-Oon KO,*1 Oh Seuk LEE,*1 Young Hun KIM,*3 Seok Han KIM,*4 Nam Ho HEO,*4 and Woo Taik LIM*1† *1 Department of Applied Chemistry, Andong National University, Andong 760–749, Korea *2 Pohang Accelerator Laboratory, Pohang University of Science and Technology, PO Box 125, Pohang 790–600, Korea *3 Department of Environmental Engineering, Andong National University, Andong 760–749, Korea *4 Department of Applied Chemistry, Kyungpook National University, Daegu 702–701, Korea –6 The crystal structure of Na71[Si121Al71O384]-FAU, a = 24.946(3)Å, dehydrated at 673 K and 1 × 10 Torr, was determined by single-crystal X-ray diffraction techniques in the cubic space group Fd3m at 294 K. About 71 Na+ ions per unit cell are found at an unusually large number of crystallographically distinct positions, seven. Five Na+ ions are at the centers of double 6-rings (D6Rs, site I). Two site-I′ positions (in the sodalite cavities opposite D6Rs) are occupied by eight and fourteen Na+ ions, respectively, per unit cell. Thirteen and fifteen Na+ ions per unit cell are found at two site-II positions, in the supercages opposite S6Rs. The remaining twelve and four Na+ ions occupy two sites III′ near triple 4- rings in the supercage. (Received April 3, 2006; Accepted June 19, 2006; Published on web August 25, 2006) This work was made to investigate the sites of Na+ in zeolite Y collected at 294(1)K on an ADSC Quantum210 detector at (synthetic faujasite (FAU) with Si/Al = 1.69) by single-crystal Beamline 4A MXW of Pohang Light Source. -

Synthesis of Zeolite Y from Kaolin Using Novel Method of Dealumination
International Journal of Applied Engineering Research ISSN 0973-4562 Volume 12, Number 5 (2017) pp. 755-760 © Research India Publications. http://www.ripublication.com Synthesis of Zeolite Y from Kaolin Using Novel Method of Dealumination 1Adeoye, J. B., 2Omoleye, J. A., 3Ojewumi, M. E. and 4Babalola, R. 1,2,3Chemical Engineering Department, Covenant University, P.M.B 1023, Ota, Ogun state, Nigeria. 4Chemical and Petrochemical Engineering Department, Akwa Ibom State University, Ikot Akpaden. Abstract fascinating chemisorptions, high selectivity and thermal In this study Zeolite Y was successfully synthesized from local stability properties [1]. Zeolites are synthesis traditionally by kaolin in Ado-0do Ota, Ogun state Nigeria through a novel hydro gel of Sodium aluminate and silicate [2]. The production process of dealumination. The thermal activation of kaolin was of zeolite from clay as a source of silica and alumina are been achieved through the process of metakaolinization at 850 oC for investigates with positive achievement [3-4]. The benefits of 6 hours in a furnace and dealumination with H2SO4 in order to using kaolin as an aluminosilicate source in zeolite production achieve a desire silica/alumina molar ratio between 3 and 8. are widely known [5]. Zeolitization involved alkaline attack of dealuminated metakaolin and its consequent transformation into Zeolite Y crystal. Silica/Alumina molar ratio of 5.84 of metakaolin was MATERIALS AND METHOD synthesized under hydrothermal treatment with aqueous NaOH Materials at atmospheric pressure. It was then aged for 7 days at room Kaolinite clay used in the course of synthesis of zeolite Y was temperature and crystallized at 100 oC for 24 hours; Zeolite procured from Arobieye village in Ado-Odo, Ota, Ogun state. -

The Surface Structure and Growth of Natural Zeolites
A thesis submitted for the degree of Doctor of Philosophy The Surface Structure and Growth of Natural Zeolites by Manisha Mistry University College London 2005 Davy Faraday Research Laboratory, The Royal Institution of Great Britain. Centre for Theoretical and Computational Chemistry, Department of Chemistry, University College London 1 UMI Number: U592160 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U592160 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 A b s t r a c t A systematic study has been carried out in order to investigate two aspects o f zeolite chemistry: to determine the external surface structure and to establish the mechanism of crystal growth. The natural zeolite edingtonite (EDI) was chosen as a model material and the surface structures were studied by finding the most stable termination for each face. For each of the four morphologically important faces the surface structure was found to minimise the number of broken bonds created upon cleaving the surface. In the absence of experimental data, the crystal morphologies were used as a proxy indicator of relative growth rate of different faces. -

→ Faujasite Growth During Palagonitisation of Mg-Rich
Faujasite Growth During Palagonitisation of Mg-rich Sideromelane: an Example from the Kaiser- stuhl Volcanic Complex, SW Germany Spurgin,¨ Simon1 Weisenberger, Tobias2 1Hans G. Hauri Mineralstoffwerk, Bergstrasse 114, 79268 Botzingen¨ 2Albert-Ludwigs-University Freiburg, Institute of Mineralogy and Geochemistry, Albertstrasse 23b, 79104 Freiburg Faujasite, [NaCa0.5Mg0.5K]x[AlxSi12−xO24] •16H2O, is a rare zeolite, only known from about ten localities, where it occurs in cavities of basaltic lavas and hydrothermally altered granitic rocks. Despite the ease of synthesizing zeolite X and zeolite Y, the industrial analogues of natural faujasite, it is astonishing that faujasite is so sparse in nature. Low-grade zeolite facies mineralization in the Kaiserstuhl volcanic complex results from subsolidus hydrothermal al- teration of alkaline volcanic rocks. Nine different zeolite species are known from the volcanic complex: analcime, chabazite-Ca, faujasite-Na, faujasite-Mg, natrolite, offretite, phillipsite-K, phillipsite-Ca and thomsonite. New micro- probe data on barrel-shaped offretite from the Limberg area clearly shows that the postulated epitaxial intergrowth of offretite and erionite (Rinaldi 1976) does not occur. Zeolite occurrence as well as their chemical composition depends on the chemical composition of the host rock, local hydrological features and porosity of the rock. In the Kaiserstuhl Volcanic Complex, octahedral crystals of faujasite occur in limburgite (olivine-augite basanite) lava flows as minor secondary mineral, associated with offretite, phillipsite, chabazite, aragonite, calcite, dolomite, montmo- rillonite and hyalite. The flows consist of a relatively well crystallised main zone, which becomes increasingly vesicular towards the vitreous, scoriaceous top. Faujasite is most abundant in these glass-rich, highly porous zones (Lorent 1933). -
Sedimentary Zeolite Deposits in Jordan
Sedimentary Zeolite Deposits in Jordan Table 1: Location of the zeolitic tuff localities in Jordan (see map) Locality Name Longitude (N) Latitude (E) 1 Tell Rimah North 32° 19' 11 36° 52' 49" 2 Tell Rimah South 32° 18' 55 36° 52' 54 3 Jabal Aritayn North 32° 04' 44 36° 51' 23 4 Jabal Aritayn South 32° 04' 36 36° 51' 37 5 Jabal Hannoun 32° 23' 15 37° 37' 44 6 Ashgof Wira 32° 17' 10” 37° 39' 34” 7 Jabal Tarboush 32° 23' 29 37° 37' 06 8 Tell Hassan 32° 01' 05 36° 37' 25 9 Ashgof North 32° 18' 18” 36° 37' 21” 10 Tall Humilan 33° 25' 20” 37° 33' 29” 11 Tall Al Boughaili 32° 26' 10” 37° 32' 40” 12 Lithyam 32° 12' 33” 37° 40' 60” 13 Jabal Ufyhim 32° 04' 44” 36° 51' 23” 14 Jabal Jalad 32° 09' 04” 36° 52' 36” 15 Wadi Zarqa Ma’in 31° 36' 09” 53° 35'49’ 16 Tall Juhira 30° 38' 47” 35° 49' 37” 17 Tall Amir 30° 37' 23” 35 ° 49' 43” 18 Al Alia 30° 33' 05” 35° 47' 58” Introduction: The Neogene-Quaternary plateau lavas of Jordan are part of the major "North Arabian Volcanic Province" which extends from Syria across Jordan into Saudi Arabia, covering in Jordan some 11,000 km2 (Ibrahim, 1993, Tarawneh et al., 2000). These lavas are predominantly alkali olivine basalts and are classified as the Harrat Ash-Shaam super-group (HASB), with a thickness from 100 m to 1500 m (Ibrahim, 1993). The HASB has an age range from 26 Ma to <0.5 Ma (Tarawneh et al., 2000). -
STRUCTURAL CLASSIFICATION of ZEOLITES Department of The
MINERALOGICAL SOCIETY OF AMERICA, SPECIAL PAPER 1, 1963 INTERNATIONAL MINERALOGICAL ASSOCIATION, PAPERS, THIRD GENERAL MEETING STRUCTURAL CLASSIFICATION OF ZEOLITES J. V. SMITH Department of the Geophysical Sciences, University of Chicago, Chicago, Illinois ABSTRACT In the past ten years the structures of bikitaite, chabazite, dachiardite, erionite, faujasite, gismondine, gmelinite, harmotome, levyne, mordenite, phillipsite and Linde A have been determined. In addition, proposals have been made for NaP1 and heulandite. Although many structures remain undetermined, it is possible to extend the structural classification started by W. L. Bragg in "Atomic Structure of Minerals" on the basis of the determinations of analcime and the natrolite group. The zeolites chabazite, gmelinite, erionite and levyne are based on different ways of linking together parallel six- membered rings. Harmotome, phillipsite, NaPl and gismondine are based on parallel four-membered rings, and are related structurally to feldspar and paracelsian. The structures of faujasite and Linde A are both based on tetrahedra lying at the corners of truncated octahedra linked by other polyhedra, and are thus related to sodalite. The columns of linked five-membered rings in mordenite and dachiardite may also form the basis of other zeolites with a 7.5 A repeat distance (laumontite and brewsterite) but probably not of ferrierite and heulandite. INTRODUCTION with a framework structure enclosing cavities occu- The most recent summary of the crystal structures pied by large ions and water molecules, both of which of zeolites is that given by W. L. Bragg in "Atomic have considerable freedom of movement, permitting Structure of Minerals" published in 1937 by Cornell ion-exchange and reversible dehydration. -
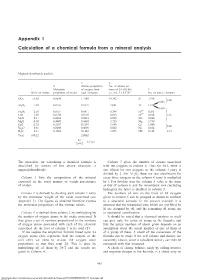
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Us Department of the Interior
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY Zeolites and selected other hydrothermal minerals in the Cascade Mountains of northern Oregon by Keith E. Bargar and Robert L. Oscarson Open-File Report 97-100 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. U.S. Geological Survey, 345 Middlefield Road, Menlo Park, CA 94025 CONTENTS Abstract........................................................ ..4 Introduction ...................................................... .4 Analytical methods ............................................. 5 Geothermal areas .............................................. 6 Breitenbush-Austin hot springs area ............................ 6 Mount Hood area ........................................ .6 Newberry volcano ........................................ 7 Zeolite minerals .................................................... 7 Analcime and Wairakite ......................................... .7 Chabazite ................................................... 8 Dachiardite .................................................. 9 Epistilbite ................................................... 9 Erionite .................................................... .9 Faujasite ................................................... .9 Ferrierite ................................................. -

FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions
materials Article FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions Ifeoma V. Joseph * , Lubomira Tosheva * , Gary Miller and Aidan M. Doyle Department of Natural Sciences, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, UK; [email protected] (G.M.); [email protected] (A.M.D.) * Correspondence: [email protected] (I.V.J.); [email protected] (L.T.) Abstract: In this research, a vermiculite-kaolinite clay (VK) was used to prepare faujasite zeolites via alkaline fusion and hydrothermal crystallisation. The optimal synthesis conditions were 1 h fusion with NaOH at 800 ◦C, addition of deionised water to the fused sample at a sample to deionised water mass ratio of 1:5, 68 h of non-agitated ageing of the suspension, and 24 h of hydrothermal treatment at 90 ◦C. The efficacy of the prepared faujasite was compared to raw clay and a reference zeolite material through adsorption experiments of aqueous solutions containing five divalent cations—Cd, Co, Cu, Pb, and Zn. The results showed that in the presence of competing cations at concentrations of 300 mg L−1 and adsorbent loading of 5 g L−1, within the first 10 min, about 99% of Pb, 60% of Cu, 58% of Cd, 28% of Zn, and 19% of Co were removed by the faujasite prepared from clay. Two to four parameter nonlinear adsorption isotherms were used to fit the adsorption data and it was found that overall, three and four parameter isotherms had the best fit for the adsorption process. -
Faujasite (Na2; Ca)Al2si4o12 ² 8H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Cubic
Faujasite (Na2; Ca)Al2Si4O12 ² 8H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Cubic. Point Group: 4=m 3 2=m: Crystals octahedra or rarely trisoctahedra, to 4 mm. Twinning: On 111 , contact and penetration twins. f g Physical Properties: Cleavage: 111 , perfect. Fracture: Uneven to conchoidal. Tenacity: Brittle. Hardness = 4.5{5f D(gmeas.) = 1.92{1.93 D(calc.) = 2.09 Optical Properties: Transparent to translucent. Color: White, colorless, brown; colorless in thin section. Streak: White. Luster: Vitreous to adamantine. Optical Class: Isotropic. n = 1.466{1.480 Cell Data: Space Group: F d3m: a = 24.638{24.65 Z = 32 X-ray Powder Pattern: San Bernardino Co., California, USA. 3.757 (100), 14.2 (95), 5.64 (83), 3.292 (64), 4.355 (52), 2.904 (36), 2.373 (36) Chemistry: (1) (2) SiO2 46.12 48.59 Al2O3 16.81 15.97 MgO 0.79 CaO 4.79 4.93 Na2O 5.09 3.02 K2O 0.16 H2O 27.02 [26.54] Total 99.83 [100.00] (1) Kaiserstuhl, Germany. (2) San Bernardino Co., California, USA; by electron microprobe, average of 15 analyses, H2O by di®erence, corresponding to (Na0:52Ca0:47Mg0:10K0:02)§=1:11 Al1:78Si4:24O12 ² 16H2O: Mineral Group: Zeolite group. Occurrence: Rare, in vesicles of basalts, phonolites, and tu®s; formed by palagonization or authigenically. Association: Zeolites, olivine, augite, nepheline. Distribution: In Germany, from Sasbach, Kaiserstuhl, and elsewhere in Baden-WuÄrttemberg; from the P°asterkaute, near Eisenach, Thuringia; at Annerod, near Giessen, and at a number of localities around the Vogelsberg volcano, Hesse.