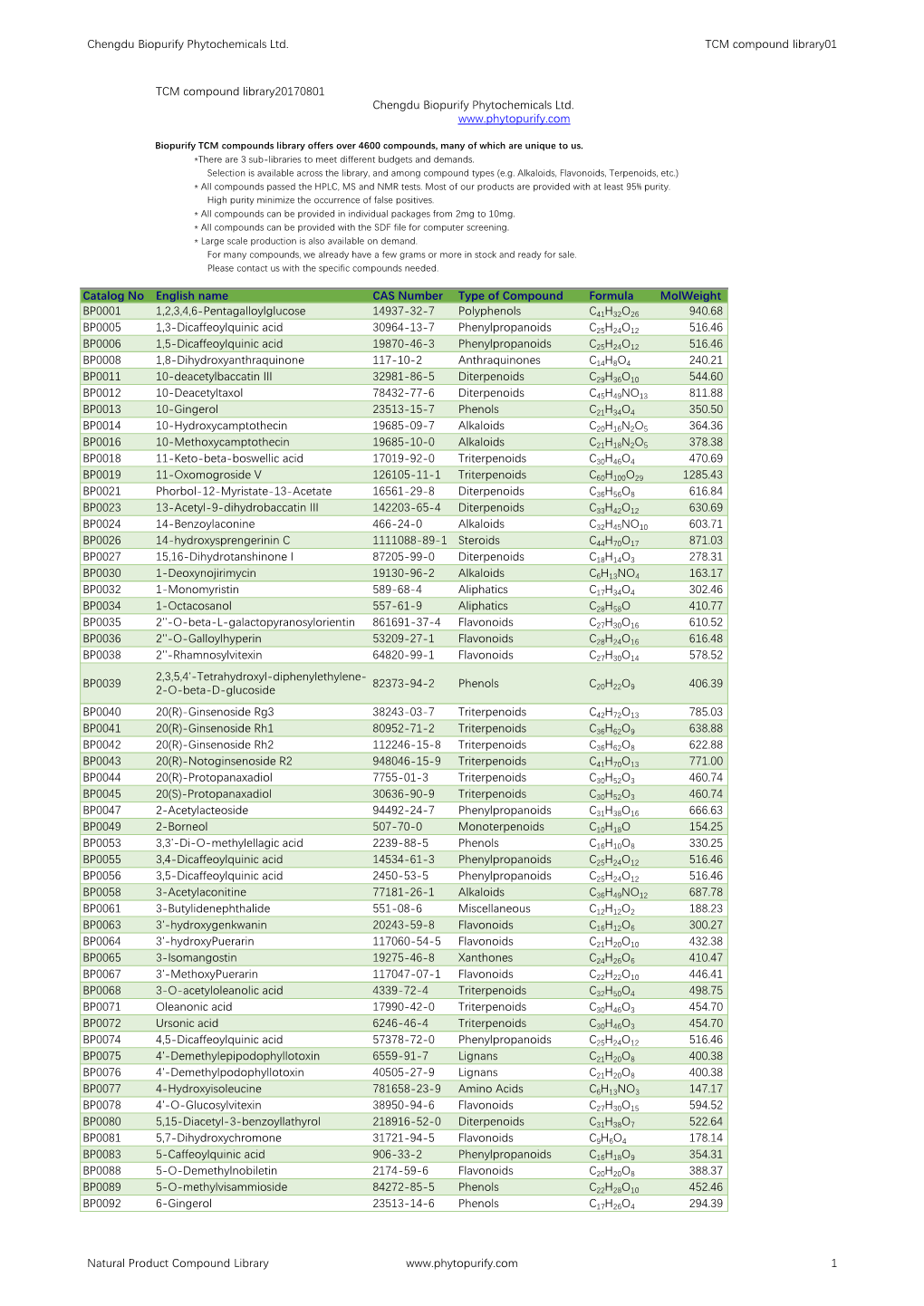
TCM Compound Library-20170801.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Bioactive Compounds in Agricultural Products and Their Roles in Health Promoting Functions
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2015 The ioB active Compounds in Agricultural Products and Their Roles in Health Promoting Functions Yixiao Shen Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Life Sciences Commons Recommended Citation Shen, Yixiao, "The ioB active Compounds in Agricultural Products and Their Roles in Health Promoting Functions" (2015). LSU Doctoral Dissertations. 2791. https://digitalcommons.lsu.edu/gradschool_dissertations/2791 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. THE BIOACTIVE COMPOUNDS IN AGRICULTURAL PRODUCTS AND THEIR ROLES IN HEALTH PROMOTING FUNCTIONS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The School of Nutrition and Food Sciences by Yixiao Shen B.S., Shenyang Agricultural University, 2010 M.S., Shenyang Agricultural University, 2012 December 2015 ACKNOWLEDGEMENTS This dissertation is a lively description of my whole Ph.D. life which is full of love from the ones who played an integral role in the completion of this degree. It is with my deepest gratitude to express my appreciation to those helping me realize my dream. To Dr. Zhimin Xu, thank you so much for offering me the opportunity to pursue my doctoral degree under your mentorship. -

Review Article Caffeates and Caffeamides: Synthetic Methodologies and Their Antioxidant Properties
Hindawi International Journal of Medicinal Chemistry Volume 2019, Article ID 2592609, 15 pages https://doi.org/10.1155/2019/2592609 Review Article Caffeates and Caffeamides: Synthetic Methodologies and Their Antioxidant Properties Merly de Armas-Ricard ,1 Enrique Ruiz-Reyes,2 and Oney Ramírez-Rodríguez 1 1Laboratory of Chemistry and Biochemistry, Campus Lillo, University of Aysén, Eusebio Lillo 667, Coyhaique 5951537, Aysén, Chile 2Department of Chemistry, Basic Sciences Institute, Technical University of Manabí (Universidad Técnica de Manabí), Av Urbina y Che Guevara, Portoviejo, Manabí, Ecuador Correspondence should be addressed to Merly de Armas-Ricard; [email protected] and Oney Ramírez-Rodríguez; [email protected] Received 29 April 2019; Accepted 25 July 2019; Published 11 November 2019 Academic Editor: Rosaria Volpini Copyright © 2019 Merly de Armas-Ricard et al. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Polyphenols are secondary metabolites of plants and include a variety of chemical structures, from simple molecules such as phenolic acids to condensed tannins and highly polymerized compounds. Caeic acid (3,4-dihydroxycinnamic acid) is one of the hydroxycinnamate metabolites more widely distributed in plant tissues. It is present in many food sources, including coee drinks, blueberries, apples, and cider, and also in several medications of popular use, mainly those based on propolis. Its derivatives are also known to possess anti-inammatory, antioxidant, antitumor, and antibacterial activities, and can contribute to the prevention of atherosclerosis and other cardiovascular diseases. is review is an overview of the available information about the chemical synthesis and antioxidant activity of caeic acid derivatives. -

Metabolic Engineering of Microbial Cell Factories for Biosynthesis of Flavonoids: a Review
molecules Review Metabolic Engineering of Microbial Cell Factories for Biosynthesis of Flavonoids: A Review Hanghang Lou 1,†, Lifei Hu 2,†, Hongyun Lu 1, Tianyu Wei 1 and Qihe Chen 1,* 1 Department of Food Science and Nutrition, Zhejiang University, Hangzhou 310058, China; [email protected] (H.L.); [email protected] (H.L.); [email protected] (T.W.) 2 Hubei Key Lab of Quality and Safety of Traditional Chinese Medicine & Health Food, Huangshi 435100, China; [email protected] * Correspondence: [email protected]; Tel.: +86-0571-8698-4316 † These authors are equally to this manuscript. Abstract: Flavonoids belong to a class of plant secondary metabolites that have a polyphenol structure. Flavonoids show extensive biological activity, such as antioxidative, anti-inflammatory, anti-mutagenic, anti-cancer, and antibacterial properties, so they are widely used in the food, phar- maceutical, and nutraceutical industries. However, traditional sources of flavonoids are no longer sufficient to meet current demands. In recent years, with the clarification of the biosynthetic pathway of flavonoids and the development of synthetic biology, it has become possible to use synthetic metabolic engineering methods with microorganisms as hosts to produce flavonoids. This article mainly reviews the biosynthetic pathways of flavonoids and the development of microbial expression systems for the production of flavonoids in order to provide a useful reference for further research on synthetic metabolic engineering of flavonoids. Meanwhile, the application of co-culture systems in the biosynthesis of flavonoids is emphasized in this review. Citation: Lou, H.; Hu, L.; Lu, H.; Wei, Keywords: flavonoids; metabolic engineering; co-culture system; biosynthesis; microbial cell factories T.; Chen, Q. -

Preventive and Therapeutic Effects of Chinese Herbal Compounds Against Hepatocellular Carcinoma
molecules Review Preventive and Therapeutic Effects of Chinese Herbal Compounds against Hepatocellular Carcinoma Bing Hu 1,*, Hong-Mei An 2, Shuang-Shuang Wang 1, Jin-Jun Chen 3 and Ling Xu 1 1 Department of Oncology and Institute of Traditional Chinese Medicine in Oncology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China; [email protected] (S.-S.W.); [email protected] (L.X.) 2 Department of Science & Technology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 202032, China; [email protected] 3 Department of Plastic & Reconstructive Surgery, Shanghai Key Laboratory of Tissue Engineering, The Ninth People’s Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200011, China; [email protected] * Correspondence: [email protected]; Tel.: +86-21-64385700 Academic Editor: Derek J. McPhee Received: 16 November 2015 ; Accepted: 20 January 2016 ; Published: 27 January 2016 Abstract: Traditional Chinese Medicines, unique biomedical and pharmaceutical resources, have been widely used for hepatocellular carcinoma (HCC) prevention and treatment. Accumulated Chinese herb-derived compounds with significant anti-cancer effects against HCC have been identified. Chinese herbal compounds are effective in preventing carcinogenesis, inhibiting cell proliferation, arresting cell cycle, inducing apoptosis, autophagy, cell senescence and anoikis, inhibiting epithelial-mesenchymal transition, metastasis and angiogenesis, regulating immune function, reversing drug -

Synthesis of Ochnaflavone and Its Inhibitory Activity on PGE 2
Synthesis and Biological Activity of Ochnaflavone Bull. Korean Chem. Soc. 2014, Vol. 35, No. 11 3219 http://dx.doi.org/10.5012/bkcs.2014.35.11.3219 Synthesis of Ochnaflavone and Its Inhibitory Activity on PGE2 Production Sung Soo Kim,† Van Anh Vo,† and Haeil Park* College of Pharmacy and †Medicine of Kangwon National University, Chuncheon 200-701, Korea *E-mail: [email protected] Received June 5, 2014, Accepted July 11, 2014 Ochnaflavone, a naturally occurring biflavonoid composed of two units of apigenin (5,7,4'-trihydroxyflavone) joined via a C-O-C linkage, was first synthesized and evaluated its inhibitory activity on PGE2 production. Total synthesis was accomplished through modified Ullmann diaryl ether formation as a key step. Coupling reactions of 4'-halogenoflavones and 3'-hydroxy-5,7,4'-trimethoxyflavone were explored in diverse reaction conditions. The reaction of 4'-fluoro-5,7-dimethoxyflavone (2c) and 3'-hydroxy-5,7,4'-trimethoxyflavone (2d) in N,N-dimethylacetamide gave the coupled compound 3 in 58% yield. Synthetic ochnaflavone strongly inhibited PGE2 production (IC50 = 1.08 µM) from LPS-activated RAW 264.7 cells, which was due to reduced expression of COX-2. On the contrary, the inhibition mechanism of wogonin was somewhat different from that of ochnaflavone although wogonin, a natural occurring anti-inflammatory flavonoid, showed strong inhibitory activity of PGE2 production (IC50 = 0.52 µM), and seems to be COX-2 enzyme inhibition. Our concise total synthesis of ochnaflavone enable us to provide sufficient quantities of material for advanced biological studies as well as to efficiently prepare derivatives for structure-activity relationship study. -

Original Article Effect of GABA on Blood Pressure and Blood Dynamics of Anesthetic Rats
Int J Clin Exp Med 2015;8(8):14296-14302 www.ijcem.com /ISSN:1940-5901/IJCEM0008622 Original Article Effect of GABA on blood pressure and blood dynamics of anesthetic rats Pengju Ma1, Ting Li2, Fanceng Ji3, Haibo Wang4, Juntao Pang4 1Department of Anesthesiogy, Anqiu People’s Hospital, Anqiu 262100, China; 2Delivery Room, People’s Hospital of Anqiu, Anqiu 262100, China; 3Department of Anesthesiogy, Weifang People’s Hospital, Weifang 261041, China; 4Department of Critical Care Medicine of Weifang People’s Hospital, Weifang 261041, China Received March 31, 2015; Accepted August 13, 2015; Epub August 15, 2015; Published August 30, 2015 Abstract: Background: This study aimed to investigate GABA effects on blood pressure and blood dynamics of an- esthetic rats by observing spontaneously hypertensive rats under both anesthesia and waking state. Materials and methods: 72 male waking Wistar-Kyokos (WKY) rats and 72 male anesthetized spontaneously hypertensive (SHR) rats were randomly divided into control group and experimental group (N = 36 each). Rats were further divided into three subgroups (N = 12 each), which received 15 μmol GABA, 35 nmol muscimol, or 4 nmol dicentrine into uni- lateral paraventricular nucleus, respectively. Rats in the control group (WKY1) and experimental group (SHR1) were compared for the GABA effect on blood pressure (MAP), heart rate (HR), and arterial baroreceptor reflex function (BRS) changes under waking state. Anesthetic WKY rats (WKY2) and spontaneously hypertensive rats (SHR2) were compared for the GABA effect on those abovementioned indexes. Abdominal aorta mean arterial pressure, heart rate, and arterial baroreceptor reflex function changes were compared in all rats. Results: MAP, HR, and BRS were slightly lower in the rats under anesthetic state than in waking state before treatment (P < 0.05); they did not show significant changes between anesthetic and waking state, however, after treatment (P > 0.05). -

Supplementary Materials Evodiamine Inhibits Both Stem Cell and Non-Stem
Supplementary materials Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70 Seung Yeob Hyun, Huong Thuy Le, Hye-Young Min, Honglan Pei, Yijae Lim, Injae Song, Yen T. K. Nguyen, Suckchang Hong, Byung Woo Han, Ho-Young Lee - 1 - Table S1. Short tandem repeat (STR) DNA profiles for human cancer cell lines used in this study. MDA-MB-231 Marker H1299 H460 A549 HCT116 (MDA231) Amelogenin XX XY XY XX XX D8S1179 10, 13 12 13, 14 10, 14, 15 13 D21S11 32.2 30 29 29, 30 30, 33.2 D7S820 10 9, 12 8, 11 11, 12 8 CSF1PO 12 11, 12 10, 12 7, 10 12, 13 D3S1358 17 15, 18 16 12, 16, 17 16 TH01 6, 9.3 9.3 8, 9.3 8, 9 7, 9.3 D13S317 12 13 11 10, 12 13 D16S539 12, 13 9 11, 12 11, 13 12 D2S1338 23, 24 17, 25 24 16 21 D19S433 14 14 13 11, 12 11, 14 vWA 16, 18 17 14 17, 22 15 TPOX 8 8 8, 11 8, 9 8, 9 D18S51 16 13, 15 14, 17 15, 17 11, 16 D5S818 11 9, 10 11 10, 11 12 FGA 20 21, 23 23 18, 23 22, 23 - 2 - Table S2. Antibodies used in this study. Catalogue Target Vendor Clone Dilution ratio Application1) Number 1:1000 (WB) ADI-SPA- 1:50 (IHC) HSP70 Enzo C92F3A-5 WB, IHC, IF, IP 810-F 1:50 (IF) 1 :1000 (IP) ADI-SPA- HSP90 Enzo 9D2 1:1000 WB 840-F 1:1000 (WB) Oct4 Abcam ab19857 WB, IF 1:100 (IF) Nanog Cell Signaling 4903S D73G4 1:1000 WB Sox2 Abcam ab97959 1:1000 WB ADI-SRA- Hop Enzo DS14F5 1:1000 WB 1500-F HIF-1α BD 610958 54/HIF-1α 1:1000 WB pAkt (S473) Cell Signaling 4060S D9E 1:1000 WB Akt Cell Signaling 9272S 1:1000 WB pMEK Cell Signaling 9121S 1:1000 WB (S217/221) MEK Cell Signaling 9122S 1:1000 -

United States Patent (19) 11 Patent Number: 6,066,311 Cheetham Et Al
US006066311A United States Patent (19) 11 Patent Number: 6,066,311 Cheetham et al. (45) Date of Patent: May 23, 2000 54 PRODUCTION AND USES OF CAFFEIC 63–284117 11/1988 Japan. ACID AND DERVATIVES THEREOF 63–284118 11/1988 Japan. 63–284119 11/1988 Japan. 75 Inventors: Peter S. J. Cheetham; Nigel E. 1013018 1/1989 Japan. Banister, both of Canterbury, United 06199649 7/1994 Japan. Kingdom 06287105 10/1994 Japan. OTHER PUBLICATIONS 73 Assignee: Zylepsis Limited, Ashford, United Kingdom Ellis, B. E., and Amrhein, N., The NIH-Shift During Aromatic OrthoHydroxylation in Higher Plants, Phytochem Appl. No.: 08/750,227 istry, 10, pp. 3069-3072, 1971, Pergamon Press. Freudenberg Karl, Berichte der Deutschen Chemischen PCT Fed: Jun. 7, 1995 Gesellschaft, vol. 53, No. 1, 1920 Weinheim DE, pp. PCT No.: PCT/GB95/01324 232-239. In German-See Ref. AK. Gestentner B., and Conn, Eric E., “The 2-Hydroxylation of S371 Date: Mar. 14, 1997 trans-Cinnamic Acid by Chloroplasts from Melilotus alba S 102(e) Date: Mar. 14, 1997 Desr.” Archives of Biochemistry and Biophysics, 163, pp. 617-624, 1974, Academic Press. 87 PCT Pub. No.: WO95/33706 Sripad, G., Prakash, V., Narasinga Rao, M.S. “Extractability of polyphenols of Sunflower Seed in various Solvents.’, J. PCT Pub. Date: Dec. 14, 1995 BioSci., 4, No. 2, pp. 145-152, 1982. 30 Foreign Application Priority Data Tranchino L., Constantino R., and Sodini G., “Food grade oilseed protein processing, Sunflower and rapeseed.”, Qual Jun. 8, 1994 GB United Kingdom ................... 941 1539 Plant Foods Hum. Nutr., 32, pp. 305-334, 1983. -

Characterization and Differentiation of Spanish Vinegars from Jerez And
foods Article Characterization and Differentiation of Spanish Vinegars from Jerez and Condado de Huelva Protected Designations of Origin Enrique Durán-Guerrero 1,* ,Mónica Schwarz 2, M. Ángeles Fernández-Recamales 3, Carmelo G. Barroso 1 and Remedios Castro 1 1 Analytical Chemistry Department—IVAGRO, Faculty of Sciences, University of Cadiz, 11510 Puerto Real, Spain 2 “Salus Infirmorum” Faculty of Nursing, University of Cadiz, 11001 Cadiz, Spain 3 Department of Chemistry, Faculty of Experimental Sciences, University of Huelva, 21007 Huelva, Spain * Correspondence: [email protected]; Tel.: +34-956-01645 Received: 23 July 2019; Accepted: 9 August 2019; Published: 12 August 2019 Abstract: Thirty one Jerez vinegar samples and 33 Huelva vinegar samples were analyzed for polyphenolic and volatile compound content in order to characterize them and attempt to differentiate them. Sixteen polyphenolic compounds were quantified by means of ultraperformance liquid chromatography method with diode array detection (UPLC–DAD), and 37 volatile compounds were studied by means of stir bar sorptive extraction–gas chromatography–mass spectrometry (SBSE–GC–MS). Spectrophotometric CIELab parameters were also measured for all the samples. The results obtained from the statistical multivariate treatment of the data evidenced a clear difference between vinegars from the two geographical indications with regard to their polyphenolic content, with Jerez vinegars exhibiting a greater phenolic content. Differentiation by the volatile compound content was, however, not so evident. Nevertheless, a considerable differentiation between the two groups of vinegars based on their volatile fraction was achieved. This may bring to light the grape varieties and geographical factors that have a clear influence on such differences. -

(12) United States Patent (10) Patent No.: US 8,980,319 B2 Park Et Al
US00898O319B2 (12) United States Patent (10) Patent No.: US 8,980,319 B2 Park et al. (45) Date of Patent: *Mar. 17, 2015 (54) METHODS OF PRODUCING STABILIZED A613 L/445 (2006.01) SOLID DOSAGE PHARMACEUTICAL A613 L/47 (2006.01) COMPOSITIONS CONTAINING A6II 45/06 (2006.01) MORPHINANS A63/67 (2006.01) (52) U.S. Cl. (71) Applicant: Mallinckrodt LLC, Hazelwood, MO CPC ............. A6 IK3I/485 (2013.01); A61 K9/1652 (US) (2013.01); A61 K9/2031 (2013.01); A61 K 9/2081 (2013.01); A61 K9/2086 (2013.01); (72) Inventors: Jae Han Park, Olivette, MO (US); A6IK9/2095 (2013.01); A61 K9/5042 Tiffani Eisenhauer, Columbia, IL (US); (2013.01); A61 K3I/4355 (2013.01); A61 K Spainty,S.Isna Gupta, F11llsborough, 31/4375A6 (2013.01); IK3I/445 gets (2013.01); it' A6 (2013.01); IK3I/47 Stephen Overholt, Middlesex, NJ (US) (2013.01); A61K 45/06 (2013.01); A61 K 9/2013 (2013.01); A61 K9/209 (2013.01); (73) Assignee: Mallinckrodt LLC, Hazelwood, MO A6 IK3I/167 (2013.01) (US) USPC ........... 424/472: 424/465; 424/468; 424/490; c - r - 514/282; 514/289 (*) Notice: Subject to any disclaimer, the term of this (58) Field of Classification Search patent is extended or adjusted under 35 N U.S.C. 154(b)b) by 0 daysyS. Seeone application file for complete search history. This patent is Subject to a terminal dis claimer. (56) References Cited (21) Appl. No.: 14/092.375 U.S. PATENT DOCUMENTS (22) Filed: Nov. 27, 2013 2008, 0026052 A1 ck 1/2008 Schoenhard ................. -

Enzyme-Site Blocking Combined with Optimization of Molecular Docking for Efficient Discovery of Potential Tyrosinase Specific In
molecules Article Enzyme-Site Blocking Combined with Optimization of Molecular Docking for Efficient Discovery of Potential Tyrosinase Specific Inhibitors from Puerariae lobatae Radix Haichun Liu 1,2,†, Yitian Zhu 1,† , Ting Wang 1, Jin Qi 1,* and Xuming Liu 3,* 1 Jiangsu key Laboratory of TCM Evaluation and Translational Research, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, China; [email protected] (H.L.); [email protected] (Y.Z.); [email protected] (T.W.) 2 School of Science, China Pharmaceutical University, Nanjing 211198, China 3 School of Life Science and Technology, China Pharmaceutical University, Nanjing 211198, China * Correspondence: [email protected] (J.Q.); [email protected] (X.L.); Tel.: +86-025-8618-5157 (J.Q.); +86-025-8618-5397 (X.L.) † These authors contributed equally to this work. Received: 1 August 2018; Accepted: 7 October 2018; Published: 11 October 2018 Abstract: Enzyme inhibitors from natural products are becoming an attractive target for drug discovery and development; however, separating enzyme inhibitors from natural-product extracts is highly complex. In this study, we developed a strategy based on tyrosinase-site blocking ultrafiltration integrated with HPLC-QTOF-MS/MS and optimized molecular docking to screen tyrosinase inhibitors from Puerariae lobatae Radix extract. Under optimized ultrafiltration parameters, we previously used kojic acid, a known tyrosinase inhibitor, to block the tyrosinase active site in order to eliminate false-positive results. Using this strategy, puerarin, mirificin, daidzin and genistinc were successfully identified as potential ligands, and after systematic evaluation by several docking programs, the rank of the identified compounds predicted by computational docking was puerarin > mirificin > kojic acid > daidzin ≈ genistin, which agreed with the results of tyrosinase-inhibition assays. -

Transcriptome Analysis of Pueraria Candollei Var
Suntichaikamolkul et al. BMC Plant Biology (2019) 19:581 https://doi.org/10.1186/s12870-019-2205-0 RESEARCH ARTICLE Open Access Transcriptome analysis of Pueraria candollei var. mirifica for gene discovery in the biosyntheses of isoflavones and miroestrol Nithiwat Suntichaikamolkul1, Kittitya Tantisuwanichkul1, Pinidphon Prombutara2, Khwanlada Kobtrakul3, Julie Zumsteg4, Siriporn Wannachart5, Hubert Schaller4, Mami Yamazaki6, Kazuki Saito6, Wanchai De-eknamkul7, Sornkanok Vimolmangkang7 and Supaart Sirikantaramas1,2* Abstract Background: Pueraria candollei var. mirifica, a Thai medicinal plant used traditionally as a rejuvenating herb, is known as a rich source of phytoestrogens, including isoflavonoids and the highly estrogenic miroestrol and deoxymiroestrol. Although these active constituents in P. candollei var. mirifica have been known for some time, actual knowledge regarding their biosynthetic genes remains unknown. Results: Miroestrol biosynthesis was reconsidered and the most plausible mechanism starting from the isoflavonoid daidzein was proposed. A de novo transcriptome analysis was conducted using combined P. candollei var. mirifica tissues of young leaves, mature leaves, tuberous cortices, and cortex-excised tubers. A total of 166,923 contigs was assembled for functional annotation using protein databases and as a library for identification of genes that are potentially involved in the biosynthesis of isoflavonoids and miroestrol. Twenty-one differentially expressed genes from four separate libraries were identified as candidates involved in these biosynthetic pathways, and their respective expressions were validated by quantitative real-time reverse transcription polymerase chain reaction. Notably, isoflavonoid and miroestrol profiling generated by LC-MS/MS was positively correlated with expression levels of isoflavonoid biosynthetic genes across the four types of tissues. Moreover, we identified R2R3 MYB transcription factors that may be involved in the regulation of isoflavonoid biosynthesis in P.