Part 3 September 2019 (Sun Pharmaceutical Industries Ltd.) HA698
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

List of Govt. Senior Secondary Schools School Boys/ Rural/ Name of Assembly Parliamentry Sr.No
List Of Govt. Senior Secondary Schools School Boys/ Rural/ Name of Assembly Parliamentry Sr.No. Name of School/Office Code Girls Urban Block Constituency Constituency Ambala 81 1 GSSS Adhoya 10 Co-Edu Rural Barara 06-MULLANA (SC) AC AMBALA 2 GSSS Akbarpur 53 Co-Edu Rural Naraingarh 03-NARAINGARH AC AMBALA 3 GSSS Alipur 70 Co-Edu Rural Barara 06-MULLANA (SC) AC AMBALA 4 GSSS Ambala Cantt (Rangia Mandi) 6 Co-Edu Urban Ambala-II (Cantt) 04-AMBALA CANTT AC AMBALA 5 GSSS Ambala Cantt. (B.C. Bazar) 4 Co-Edu Urban Ambala-II (Cantt) 04-AMBALA CANTT AC AMBALA 6 GSSS Ambala Cantt. (Bakra Market) 5 Co-Edu Urban Ambala-II (Cantt) 04-AMBALA CANTT AC AMBALA 7 GSSS Ambala Cantt. (Main Branch) 171 Co-Edu Urban Ambala-II (Cantt) 04-AMBALA CANTT AC AMBALA 8 GSSS Ambala Cantt. (Ram Bagh 7 Co-Edu Urban Ambala-II (Cantt) 04-AMBALA CANTT AC AMBALA Road) 9 GSSS Ambala City (Baldev Nagar) 8 Co-Edu Urban Ambala-I (City) 05-AMBALA CITY AC AMBALA 10 GGSSS Ambala City (Baldev Nagar) 69 Girls Urban Ambala-I (City) 05-AMBALA CITY AC AMBALA 11 GGSSS Ambala City (Model Town) 172 Girls Urban Ambala-I (City) 05-AMBALA CITY AC AMBALA 12 GGSSS Ambala City (Police Line) 143 Girls Urban Ambala-I (City) 05-AMBALA CITY AC AMBALA 13 GSSS Ambala City (Prem Nagar) 9 Co-Edu Urban Ambala-I (City) 05-AMBALA CITY AC AMBALA 14 GSSS Babyal 11 Boys Urban Ambala-II (Cantt) 04-AMBALA CANTT AC AMBALA 15 GSSS Badhauli 14 Co-Edu Rural Naraingarh 03-NARAINGARH AC AMBALA 16 GSSS Baknaur 71 Co-Edu Rural Ambala-I (City) 05-AMBALA CITY AC AMBALA 17 GSSS Ballana 12 Co-Edu Rural Ambala-I (City) -

Sun Pharma Science Foundation's Annual Conference and Research
(Registered under the Societies Registration Act XXI of 1860) Sarhaul, Sector-18, Gurgaon-122015 (Haryana), INDIA. Phone : (91-124) 2341477 (D); 4194342; 4194400; 4194200 E-mail : [email protected] FOR IMMEDIATE RELEASE Sun Pharma Science Foundation recognizes Indian scientists for exemplary contribution in pharma & medical science New Delhi – February 13, 2018: Sun Pharma Science Foundation, a non-profit organization registered under Societies Registration Act announced the Sun Pharma Science Awards to Indian Scientists for their outstanding work and exemplary contribution to medical research. These awards are presented in two categories - The Sun Pharma Research Awards for outstanding scientists and Sun Pharma Science Scholar Awards for young researchers. The winners for both these awards are identified in two sub-categories - Medical Sciences and Pharmaceutical Sciences. An eminent jury panel comprising well-known scientists from India selected the final winners. These Awards are presented annually to Indian scientists & young researchers working in India and abroad. The awards were presented by Prof. Vishwajit Nimgaonkar, Professor of Psychiatry and Human Genetics at the University of Pittsburgh, USA and Mr. Dilip Shanghvi, Managing Director, Sun Pharma. Sun Pharma Research Award Winners for 2016 Medical Sciences – Basic Research Award Winner Dr. Rajan Sankaranarayanan Chief Scientist CSIR-Centre for Cellular and Molecular Biology Uppal Road, Hyderabad – 500 007, India Dr. Sankaranarayanan receives this award for his outstanding contributions in the area of protein biosynthesis, by studying proofreading mechanisms using structural biology approaches. Registered Office : 8-C, 8th Floor, Hansalaya Building, 15-Barakhamba Road, Connaught Place, New Delhi-110001 (Registered under the Societies Registration Act XXI of 1860) Sarhaul, Sector-18, Gurgaon-122015 (Haryana), INDIA. -

S!Jw· Chief General Manager & Company Secretary
MARUTI �$SUZUKI Way ofLIia! MSIL: CSL: NSE&BSE: 2018 +� A ) 2C3 u�beY2018 Vice President General Manager National Stock Exchange of India Limited Department of Corporate Services "Exchange Plaza", Bandra- Kurla Complex BSE Limited Bandra (E) Phiroze Jeejeebhoy Towers Mumbai - 400 051 Dalal Street, Mumbai - 400 001 Sub: Press Release Dear Sir, Please find enclosed herewith as Annexure -"A", a copy of the press release being issued today. Kindly take the same on record. Thanking you, Yours truly, <ForMaruti Suzuki India Limited s!jW· Chief General Manager & Company Secretary Encl.: As above MARUTI SUZUKI INDIA LIMITED CIN:L34103DL1981PLC011375 Registered & Head Office Gurgaon Plant: Manesar Plant: Maruti Suzuki India Limited, Maruti Suzuki India Limited, Maruti Suzuki India Limited, 1 Nelson Mandela Road, Vasant Kunj, Old Palam Gurgaon Road, Plot No.1, Phase 3A, IMT Manesar, New Delhi 110070, India. Gurgaon 122015, Haryana, India. Gurgaon 122051, Haryana, India. Tel: 011-46781000, Fax: 011-46150275/46150276 Tel. 0124-2346721, Fax: 0124-2341304 Tel: 0124-4884000, Fax: 0124-4884199 www.marutisuzuki.com E-mail: [email protected], [email protected] Annexure-“A” PRESS RELEASE Maruti Suzuki sets up Water ATM in Sarhaul village o Company plans to install eight more Water ATMs in next one year; six of these will be in Haryana o Clean drinking water at 35 paisa/litre only o Built on a self-sustainable model o 10-stage UV filtration technology ensures water meets WHO standards, retains essential minerals Gurugram, 29th October 2018: Access to clean drinking water has been identified as critical need by Maruti Suzuki in its adopted villages. -

Actemra Customer List
Actemra Customer List Description District Name 1 Street City Postal Code E-Mail Address Telephone Maharashtra THANE A.N. PHARMA G1/G2, KANTI EMPIRE,LAL GODOWN, VASAI (W) 401202 [email protected] 9028088463 COLLEGE ROAD,VASAI (W),THANE.LBT NO 67 343 2011 Maharashtra MUMBAI ATOR HEALTHCARE PVT. LTD. SONMUR APARTMENTS,DARUWALLA MALAD (W) 400064 [email protected] 022-28662979 / 8 COMPOUND,S.V.ROAD,MALAD (W), MUMBAI Maharashtra PUNE AAKANKSHA LOGISTICS PRIVATE LTD 1ST FLOOR,C\O MANTRI HOSPITALSURVEY PUNE 411036 [email protected] 9822007606 NO 69 15.B.T. KAWADE ROADGHORPADIPUNE Andra Pradesh KRISHNA DISTRICT AUROBINDO DRUGS D.NO:8-72, BLOCK-IIPRASAD VIJAYAWADA 520007 [email protected] 0866-3202242 PLAZA,KAMAIAHTHOPU CENTER,M.G. ROADVIJAYAWADA Maharashtra WASHIM AJAY AGENCIES PATNI CHOWK,WASHIMWASHIM WASHIM 444505 [email protected] 07252 - 234699 2 Maharashtra JALGAON ANAND AGENCIES (CHQ) C.S NO 3297/A/11A, ANAND NIWAS BHUSAWAL 425201 [email protected] 02582-225569/ FIRSNEAR PANDURANG TALKIES JAMNER RDBHUSAWAL 425201 Maharashtra AURNGABAD ANIL MEDICAL STORES SHOP NO.01, HOUSE NO.3-10-53 CST AURNGABAD 431001 [email protected] 9890053040 NOGANDHI CHOWK, AURNGABAD.TAL AURNGABAD DIST. AURANGABADPIN : 431 001 Maharashtra SOLAPYR APTE AGENCIES, (CHQ) 729/3/4 CHATRAPATI COLONYKURDUWADI BARSHI 413401 [email protected] 02184-222543/ 90 ROADBARSHIDIST SOLAPUR Maharashtra YAVATMAL APEX PHARMA HALL NO. 3 , 2 ND FLOORNAGAR PARISHAD YAVATMAL 445001 [email protected] 07232-250317 25 COMPLEX,DATTA CHOWK,YAWATMAL Gujarat AHMEDABAD ANGI AGENCY GF-39,STATION ROADSHIVGANGA AHMEDABAD 382220 [email protected] 9825931530 COMPLEXBALVA Gujarat SURAT A.B.C. DISTRIBUTORS 401/402/403SURAT DAWA SURAT 395004 [email protected] 9825861920 BAZARVASTADEVDI RDKATARGAMSURAT Gujarat JAMNAGAR AMAR ENTERPRISE IST FLOOR, MINAL SHOPPING JAMNAGAR 361005 [email protected] 288-2557207/6455001 CENTRESUMAIR CLUB RD,JAMNAGARTELE 0288 2557207 2550151(R)02882563127 Gujarat GANDHINAGAR ANKUR DISTRIBUTORS 8. -

Circle Rates
g.r.p....) Year 2oL8-2or9 w.e.f. -Q-T:Q. Year of 2OL7-2OL8 Rates for the Year of 2OL8-2OL9 Rates for the year 2OL6-2OL7 Rates for the Rates of Land upto 2 Acre depth from NH-48, l\lDD lirraroam-(ahare .rveeAazA 1\oAl Mainr .rt rr, vur u6. -"vt '!!erv. District Road 10% Residentiol Commercial Agriculture Residentiol Commercial Sr. Name of Village Agriculture Residentiol Commercidl Agriculture Major District Road / Land (Rs. Per (Rs. Per Sq. Rs. Per Sq. NH-48, NPR, Gurugram No. Land (Rs. Per (Rs. Per Sq. Land (Rs. Per (Rs" Per 5q. State Highway Sohna Road Acre) Yards.) Acre) Yards.) Acrel Yards.) Yards.l 14800 3130C 18139000 27000 4200c 25% NPR 22673750 NA NA 1 Gurgaon Village 1870000c 15300 32300 18139000 2040000c 15300 32300 20400000 15300 3230C 2 lnayatpur 20400000 17000 4200c NA NA NA NA na na na 17000 42000 NA NA NA NA 3 Hidavatpur Chawani na na na na 31300 4 Sarhaul 22950000 15300 3230C 2226L500 14800 2226150C 18000 44000 25% NH-48 27826875 10% STHW 24487650 18100 3460C 5 Dundahera 22950000 18700 3570C 22261500 2226L50C 19800 4r'}OOO 25% NH-48 27826875 10% STHW 24487650 212nn 6 Moalhera 22950000 15300 32300 2226L504 14900 2226L50C i7000 4ZUUU NA NA NA NA 26400 7 Nathupur 22950000 14450 27200 2226150C 14100 2226L50C 4500c 60000 NA NA NA NA 36700 8 Sikanderpur Ghosi 20400000 15300 37400 1999200C 15000 19992000 s000c 78000 NA NA NA NA 1500c 3170C 9 Shahpur 2295000C 15300 32300 2249LOOC 2249LOOO 1800c 44000 25% NH-48 28LL3750 NA NA t796C 10 Bajghera 16150000 8s0c 18700C 15504000 820C 15504000 1500c 3s000 NA NA NA NA 12200 22000 11 SaraiAlawardi -

DBA-VOTING-LIST-2018.Pdf
Sl.No. Name Address Bar Council Bar No. Phone Phone T.U.P. H.No-322/14, Jacubpura, 1 A.L.Sahni P/1007/1983 2 2321473 9313401837 Gurgaon H.No-763-A, Ist Floor, 2 Aakash Aggarwal Block-H, Palam Vihar, P/695/1999 4 9350840785 9910991353 Gurgaon. V.P.O. Dhunela, Post 3 Aakil Ali P/3239/2014 4051 9050300395 9813313823 Office Sohna, Gurgaon H.No-249,Sector-17-A 4 Aarti Bhalla P/2016/2015 4246 9350320004 Gurgaon. H.No-224/7, H.B. 5 Aarti Hans Colony, Sec-7 Ext, P/1398/2011 3061 9910383914 Gurgaon Vill- Udaka, PO-Sohna, 6 Aazad P/3601/2009 2544 9711115701 Distt- Gurgaon Gandhi Nagar Ward No. 7 Abdul Hamid 8 Opp Mewat Model P/778/1994 4054 8860481185 School Tauru. Vill-Rewasan, Teh -Nuh, 8 Abdul Rehman P/95/1998 6 9416256151 9813039030 Distt-Gurgaon PWO Housing Complex, B-2/301, Sector 43, 9 Abha Sinha Sushant Lok-I, Gurgaon P/1800/1999 4751 9871957449 At(P) C-6/6360, Vasant Kunj, New Delhi H.No-645/20, Gali No-6, 10 Abhai singh Yadav P/514/1977 3100 9250171144 Shivji Park, Gurgaon Abhey singh H.No.1434, Sec-15, Part- 0124- 11 P/1132/2001 8 9811198319 Dahima II, Gurgaon. 6568432 Resi-Vill-Dhanwa Pur, Abhey Singh 12 Po-Daulta Bad, Distt- P/857/1995 9 9811934070 Dahiya Gurgaon H.No-209/4, Subhash 13 Abhey Singla P/420/2007 2008 9999499932 9811779952 Nagar, Gur. H.No. 225, sector 15, 14 Abhijeet Gupta P/4107/2016 4757 9654010101 Part-I, Gurgaon. -

Haryana State Council for Child Welfare 2010-11
HARYANA STATE COUNCIL FOR CHILD WELFARE 2010-11 Complete Postal Address of Crèche Units Friends Colony, Model Town, Ambala City 095518321 Prem Nagar, Near Gurudwara, A/City0171-2552425 Behind Bus Stop, Village Mallour 09896173951 Panchyat Dharamshalla, Vill. Jalbhera Panchyat Building, Vill. Mastpur 0171-2858910 Ram Nagar, A.City 09315859942 Moti Nagar, A/City09466506334 Kaith Majri, Near Sheesh Ganj Gurudwara Village Bhurangpur 0171-2857530 Baldev Nagar, A/City 09896439414 Creche Baldev Nagar, A/City 0171-2556537 ® Panchyat Dharamshalla, Near Gurudwara, Vill. Brara 09315666453 Panchyat Dharamshalla, Vill. Ugala 09896809586 Community Centre, Housing Board Colony, A/Cantt. 09729486678 Dina ki Mandi, Dharamshalla, Kumhar Mandi, A/Cantt. Community Centre, Village Mandhour.0171-2540124-R Dharamshalla, Village Sultanpur Barrier 0171-2542003 Public Library, Water Tanki, Mahesh Nagar, A/Cantt.09466596553 Dharamshalla, Near Govt. S.S. School, Vill. Babyal 0171-2663904 -R Bal Bhawan, A/City 0171-2556751 (B.B) Bal Bhawan, A/Cantt. 09466662477 Gujoani, Bhiwani Ward No. 10, Bhwani Khera, Bank Colony Bank Colony, Bhiwani M.C. Colony, Guwar Factory, Bhiwani Near Nand Trust Puccawala, Ward No. 4, Tosham, Bhiwani Near Sanatan Dharm MandirPanchyati Dharmshalla Tosham-II B.R.C.M School Kaunt Road, Bhiwani Bhootoka Mandir,Ward No. 2, Panchyati Dharmshall, Bhiwani Near Bal Udhyan Public School, Vidhya Nagar, Bhiwani Khatio Ki Dharmshall, Chang, Bhiwani Room No. 13, B.T.M Chowk, Labour Colony,, Bhiwani Near Oil Mill, Gali No. 4,Brijwasi Colony, Bhiwani # 25/50,Telewara, Bhiwani Jeetu Patit, Pawan School, Shanti Nagar, Bhiwani B.T.M Chowk, Room No. 14,Labour Colony, Bhiwani Balmiki Basti Dharmshalla, Ward No. 14, Dadri, Bhiwani Near B.T.M Mill, Main Gate, Bhiwani Indira Lane, T.I.T. -

Name of S/DIVN. DEF COUNT DHBVN ARGROSS TOP 100
S/Divn & Category Wise TOP 100 Defaulters for the month of NOV-13 under Gurgaon Circle Name of S/DIVN. DEF COUNT DHBVN ARGROSS TOP 100 DEFAULTERS ARGROSS TOP 100 DEFAULTERS ARGROSS IN % AP METER AP UNMETER BULK HT BULK LT DS FISHER GAUSHALA HORTICULTURE HT LT NDS PUBLIC WATER WORKS STREET LIGHT TEMP DS TEMP NDS IDC 18541 120380982.1 74701001.73 62.05 70584.17 0 40922.38 0 13774836.69 0 0 0 19047222.08 15666634.13 24359586.07 0 0 910817.38 830398.83 NEW COLONY 23625 71223443.94 46497948.6 65.28 262202.58 0 5060.22 0 9486878.06 0 0 0 20510785.79 9092552.91 6209838.59 0 0 351112.33 579518.12 KADIPUR 18792 293420719.6 255607070.2 87.11 73510.05 0 138981.53 0 12225013.51 0 0 0 147406743.3 37203614.3 53731157.7 0 0 912684.14 3915365.65 TOTAL Divn 60958 485025145.6 376806020.5 77.69 406296.8 0 184964.13 0 35486728.26 0 0 0 186964751.2 61962801.34 84300582.36 0 0 2174613.85 5325282.6 DLF 15674 126683717.3 94163986.07 74.33 0 0 42378080.48 0 21263272.92 0 0 0 46561.61 734472.66 28040990.55 0 0 221247.63 1479360.22 BADSHAHPUR 7618 139322394.2 59887379.46 42.98 3495572 3255076 0 0 10146110.34 0 0 61642 26356 7962203.05 16976585.07 9051194 1330433 7582208 0 SECTOR-18 MARUTI 22202 164259889.1 115435402.7 70.28 842793.29 0 850374.46 0 9865192.8 0 0 0 53009296.8 14069950.02 35519915.5 0 0 1098921.4 178958.38 SOUTH CITY 25795 146841186.3 98032754.2 66.76 399115.14 0 11149597.31 0 6303419.72 0 0 0 594045.86 1116757.52 76342650.32 0 0 568970.76 1558197.57 TOTAL Divn 71289 577107186.8 367519522.4 63.68 4737480.43 3255076 54378052.25 0 47577995.78 0 0 61642 -

Cissampelos Pareira Linn: Natural Source of Potent Antiviral Activity Against All Four Dengue Virus Serotypes
RESEARCH ARTICLE Cissampelos pareira Linn: Natural Source of Potent Antiviral Activity against All Four Dengue Virus Serotypes Ruchi Sood1¤a, Rajendra Raut2, Poornima Tyagi2, Pawan Kumar Pareek1¤a, Tarani Kanta Barman1¤a, Smita Singhal1¤a, Raj Kumar Shirumalla3¤a, Vijay Kanoje3¤a, Ramesh Subbarayan3¤a, Ravisankar Rajerethinam3¤a, Navin Sharma1¤b, Anil Kanaujia1¤c, Gyanesh Shukla1¤a, Y. K. Gupta4, Chandra K. Katiyar1¤d, Pradip K. Bhatnagar1¤e, Dilip J. Upadhyay1¤a, Sathyamangalam Swaminathan2¤f*, Navin Khanna2* 1 Department of Microbiology, New Drug Discovery Research, Ranbaxy Research Laboratories, Gurgaon, Haryana, India, 2 Recombinant Gene Products Group, International Centre for Genetic Engineering and Biotechnology, New Delhi, India, 3 Department of Pharmacology, New Drug Discovery Research, Ranbaxy Research Laboratories, Gurgaon, Haryana, India, 4 Department of Pharmacology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India ¤a Current address: Daiichi Sankyo India Pharma Private Limited, Village Sarhaul, Gurgaon, Haryana, India ¤b Current address: VLCC Personal Care Limited, Gurgaon, India ¤ OPEN ACCESS c Current address: R & D Ayurvet Limited, Village Katha, Baddi, Himanchal Pradesh, India ¤d Current address: Emami Limited, Kolkata, India Citation: Sood R, Raut R, Tyagi P, Pareek PK, ¤e Current address: Exton, Pennsylvania, United States of America Barman TK, Singhal S, et al. (2015) Cissampelos ¤f Current address: Virology Laboratory, Department of Biological Sciences, Birla Institute of Science and pareira Linn: Natural Source of Potent Antiviral Technology-Pilani, Hyderabad Campus, Jawahar Nagar, Shamirpet, Hyderabad, India * Activity against All Four Dengue Virus Serotypes. [email protected] (SS); [email protected] (NK) PLoS Negl Trop Dis 9(12): e0004255. doi:10.1371/ journal.pntd.0004255 Editor: David W.C. -
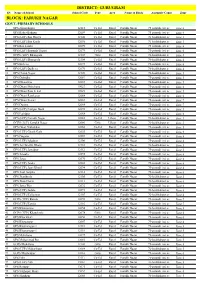
Gurugram Block: Farukh Nagar
DISTRICT: GURUGRAM SN Name of School School Code Type Area Name of Block Assembly Const. Zone BLOCK: FARUKH NAGAR GOVT. PRIMARY SCHOOLS 1 GPS Alimudinpur 12115 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 6 2 GPS Babra Bakipur 12089 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 4 3 GPS (LEP) Bas Hariya 12100 Co-Ed Rural Farukh Nagar 76-badshahpur ac Zone 3 4 GPS (LEP) Bas Kusla 12098 Co-Ed Rural Farukh Nagar 76-badshahpur ac Zone 3 5 GPS Bas Lambi 12099 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 6 6 GPS (LEP) Basunda Tirpari 12079 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 6 7 GGPS (LEP) Bhangrola 12103 Girls Rural Farukh Nagar 76-badshahpur ac Zone 4 8 GPS (LEP) Bhangrola 12104 Co-Ed Rural Farukh Nagar 76-badshahpur ac Zone 4 9 GPS Birhera 12073 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 6 10 GPS (LEP) Budhera 12070 Co-Ed Rural Farukh Nagar 76-badshahpur ac Zone 4 11 GPS Chand Nagar 12109 Co-Ed Rural Farukh Nagar 76-badshahpur ac Zone 5 12 GPS Dabodha 12081 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 5 13 GPS Dhanawas 12057 Co-Ed Rural Farukh Nagar 76-badshahpur ac Zone 5 14 GPS Dhani Mehchana 19027 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 6 15 GPS Dhani Ram Ji Lal 19029 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 5 16 GPS Dhani Ramkaran 12088 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 6 17 GPS Dhani Siwari 12053 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 7 18 GPS Dooma 12064 Co-Ed Rural Farukh Nagar 75-pataudi (sc) ac Zone 7 19 GPS (LEP) Fajilpur Badli 12090 Co-Ed Rural Farukh Nagar -

Annual Report 2014”
Prayatna ‘School of Excellence’ Redefining Indian Education.. All India Registered Society “ANNUAL REPORT 2014” Website: www.prayatnasoe.org Facebook: www.facebook.com/prayatnasoe Twitter: www.twitter.com/prayatnasoe Operational areas: Near Govt. School of Molahera, Sector 22B, Gurgaon – 122015 “Old Balmiki Chaupaal”, Village Jharsa, Near Sector 32, Gurgaon – 122015 Contact @ 0124-4228862, 9999314245, 9460022394 or write us @ [email protected] President’s Note Dear Friends, Season’s greetings!! The Annual Report of year 2014 is in your hands. I warmly welcome the new year to all of you. A new year marks a new beginning, an opportunity for renewal, change, refocus, re-energising and rejuvenation. I take pride in congratulating the team of Prayatna for their wonderful commitments and achievements in year 2014. Ever since we started PRAYATNA in January 2009, we have studied the processes and engagements of the organization- it is important to be well versed with the organization one heads. The year 2014 has seen PRAYATNA focus strongly on catering to the needs of the senior students of the government schools and enrollments of little angels into nearest govt. schools as well. Friends, I consider this to be the most crucial area of work and soon we will be assessing the works and achievements of PRAYATNA as a team. This year will always be a benchmark in the history of Prayatna as we have completed five glorious years of promoting reforms in education and we have reached over 500 children in 4 other urban rural areas of Gurgaon i.e. village Sarhaul, Jharsa, Chakarpur and Nathupur. We also touched student’s base of colleges in Gurgaon and Jaipur, Rajasthan. -

Cipremi Customer List
Cipremi Customer List Description District Name 1 Street City Postal Code E-Mail Address Telephone Maharashtra THANE A.N. PHARMA G1/G2, KANTI EMPIRE,LAL GODOWN, VASAI (W) 401202 [email protected] 9028088463 COLLEGE ROAD,VASAI (W),THANE.LBT NO 67 343 2011 Maharashtra MUMBAI ATOR HEALTHCARE PVT. LTD. SONMUR APARTMENTS,DARUWALLA MALAD (W) 400064 [email protected] 022-28662979 / 8 COMPOUND,S.V.ROAD,MALAD (W), MUMBAI Maharashtra PUNE ARHAM PHARMACEUITICALS SHOP NO 1 , LAXMI NIVAS SOCIETY893 PUNE 411030 [email protected] 020-24484637 - 9 SADASHIV PETHPUNEPUNE Maharashtra PUNE AAKANKSHA LOGISTICS PRIVATE LTD 1ST FLOOR,C\O MANTRI PUNE 411036 [email protected] 9822007606 HOSPITALSURVEY NO 69 15.B.T. KAWADE ROADGHORPADIPUNE Gujarat JAMNAGAR ASHISH MARKETING ASHISH CHAMBER,POST OFFICE JAMKHAMBALIA 361305 [email protected] 02833-232323 / 9825715921 ROAD,JAM-KHAMBHALIATAL KHAMBHALIA (JAMNAGAR) Chhaattisgarh D.C.H.COMPLEX,RAIPU ANIL MEDICAL AGENCIES D.C.H.COMPLEX,RAIPUR RdDHAMTARI DHAMTARI 493773 493773 [email protected] 9827902950 R ROAD 493773493773 Madhya Pradesh DAMOH ANIL MEDICOSE (DAMOH) 17, SHASTRI MARKETKACHORA DAMOH 470661 [email protected] 07812-221707 400707 SHOPPING CENTREDAMOHDAMOH (M.P.) Madhya Pradesh BARWANI ASHA AGENCIES (BARWANI) GAJANAND PLAZARAJGHAT BARWANI 451551 [email protected] 07290-223403 223402 ROADBARWANIBARWANI (M.P.) Andra Pradesh KRISHNA DISTRICT AUROBINDO DRUGS D.NO:8-72, BLOCK-IIPRASAD VIJAYAWADA 520007 [email protected] 0866-3202242 PLAZA,KAMAIAHTHOPU CENTER,M.G. ROADVIJAYAWADA Chhaattisgarh RAIGARH ASHOK MEDICOSE BEHIND AGRASEN BHAWANKHARSIADIST- KHARSIA 496661 [email protected] 07762-27215027313194255 RAIGARH Madhya Pradesh SEONI ARUN PHARMATICS SEONI CHHOTI POLICE LINESEONISEONISEONI SEONI 480661 [email protected] 07692-220693 (M.P.) Madhya Pradesh MHOW A.S.