Selected Properties of Vicriviroc **In October 2005, Schering Plough
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Article PDF/Slides
Kan Lu, PharmD New Antiretrovirals for Based on a presentation at prn by Roy M. Gulick, md, mph the Treatment of HIV: Kan Lu, PharmD | Drug Development Fellow University of North Carolina School of Pharmacy Chapel Hill, North Carolina The View in 2006 Roy M. Gulick, md, mph Reprinted from The prn Notebook® | october 2006 | Dr. James F. Braun, Editor-in-Chief Director, Cornell Clinical Trials Unit | Associate Professor of Medicine, Meri D. Pozo, PhD, Managing Editor. Published in New York City by the Physicians’ Research Network, Inc.® Weill Medical College of Cornell University | New York, New York John Graham Brown, Executive Director. For further information and other articles available online, visit http://www.prn.org | All rights reserved. ©october 2006 substantial progress continues to be made in the arena of cokinetics and a long extracellular half-life of approximately 10 hours antiretroviral drug development. prn is again proud to present its annual (Zhu, 2003). During apricitabine’s development, a serious drug interac- review of the experimental agents to watch for in the coming months and tion with lamivudine (Epivir) was noted. Although the plasma years. This year’s review is based on a lecture by Dr. Roy M. Gulick, a long- concentrations of apricitabine were unaffected by coadministration of time friend of prn, and no stranger to the antiretroviral development lamivudine, the intracellular concentrations of apricitabine were reduced pipeline. by approximately sixfold. Additionally, the 50% inhibitory concentration To date, twenty-two antiretrovirals have been approved by the Food (ic50) of apricitabine against hiv with the M184V mutation was increased and Drug Administration (fda) for the treatment of hiv infection. -

Download Article PDF/Slides
New Antiretrovirals in Development: Reprinted from The PRN Notebook,™ june 2002. Dr. James F. Braun, Editor-in-Chief. Tim Horn, Executive Editor. Published in New York City by the Physicians’ Research Network, Inc.,® John Graham Brown, Executive Director. For further information and other articles The View in 2002 available online, visit http://www.PRN.org All rights reserved. © june 2002. Roy “Trip” Gulick, md, mph Associate Professor of Medicine, Weill Medical College of Cornell University Director, Cornell Clinical Trials Unit, New York, New York Summary by Tim Horn Edited by Scott Hammer, md espite the fact that 16 antiretro- tiviral activity of emtricitabine was estab- Preliminary results from two random- virals are approved for use in the lished, with total daily doses of 200 mg or ized studies—FTC-302 and FTC-303—were United States, there is an indis- more producing the greatest median viral reported by Dr. Charles van der Horst and putable need for new anti-hiv com- load suppression: 1.72-1.92 log. Based on his colleagues at the 8th croi, held in Feb- pounds that have potent and these data, a once-daily dose of 200 mg ruary 2001 in Chicago (van der Horst, durable efficacy profiles, unique re- was selected for further long-term clinical 2001). FTC-302 was a blinded comparison sistance patterns, patient-friendly dosing study. “This is what we’re looking forward of emtricitabine and lamivudine, both in schedules, and minimal toxicities. To pro- to with emtricitabine,” commented Dr. combination with stavudine (Zerit) and vide prn with a glimpse of drugs current- Gulick. -

Review CCR5 Antagonists: Host-Targeted Antivirals for the Treatment of HIV Infection
Antiviral Chemistry & Chemotherapy 16:339–354 Review CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection Mike Westby* and Elna van der Ryst Pfizer Global R&D, Kent, UK *Corresponding author: Tel: +44 1304 649876; Fax: +44 1304 651819; E-mail: [email protected] The human chemokine receptors, CCR5 and suggest that these compounds have a long plasma CXCR4, are potential host targets for exogenous, half-life and/or prolonged CCR5 occupancy, which small-molecule antagonists for the inhibition of may explain the delay in viral rebound observed HIV-1 infection. HIV-1 strains can be categorised by following compound withdrawal in short-term co-receptor tropism – their ability to utilise CCR5 monotherapy studies. A switch from CCR5 to (CCR5-tropic), CXCR4 (CXCR4-tropic) or both (dual- CXCR4 tropism occurs spontaneously in approxi- tropic) as a co-receptor for entry into susceptible mately 50% of HIV-infected patients and has been cells. CCR5 may be the more suitable co-receptor associated with, but is not required for, disease target for small-molecule antagonists because a progression. The possibility of a co-receptor natural deletion in the CCR5 gene preventing its tropism switch occurring under selection pressure expression on the cell surface is not associated by CCR5 antagonists is discussed. The completion with any obvious phenotype, but can confer of ongoing Phase IIb/III studies of maraviroc, resistance to infection by CCR5-tropic strains – the aplaviroc and vicriviroc will provide further insight most frequently sexually-transmitted strains. into co-receptor tropism, HIV pathogenesis and The current leading CCR5 antagonists in clinical the suitability of CCR5 antagonists as a potent development include maraviroc (UK-427,857, new class of antivirals for the treatment of HIV Pfizer), aplaviroc (873140, GlaxoSmithKline) and infection. -

Product Monograph for CELSENTRI
PRODUCT MONOGRAPH PrCELSENTRI maraviroc Tablets 150 and 300 mg CCR5 antagonist ViiV Healthcare ULC 245, boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: July 05, 2019 Submission Control No: 226222 © 2019 ViiV Healthcare group of companies or its licensor. Trademarks are owned by or licensed to the ViiV Healthcare group of companies. Page 1 of 60 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION.........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE..............................................................................3 CONTRAINDICATIONS ...................................................................................................3 WARNINGS AND PRECAUTIONS..................................................................................4 ADVERSE REACTIONS....................................................................................................9 DRUG INTERACTIONS ..................................................................................................19 DOSAGE AND ADMINISTRATION..............................................................................28 OVERDOSAGE ................................................................................................................31 ACTION AND CLINICAL PHARMACOLOGY ............................................................31 STORAGE AND STABILITY..........................................................................................36 -

Microbicides 2008
State of the Network Ian McGowan & Sharon Hillier Annual Meeting March 14th, 2016 HIV Infection in the US HIV Infection in US MSM HIV Infection in US Women Global HIV Infection HIV Incidence Rates in ASPIRE & RING Studies ASPIRE Study RING Study 10 8 6 4 2 HIV Incidence Rates (%) Rates Incidence HIV 0 <21 years > 25 years 18-21 years22-26 years27-45 years 21-25 years The PrEP Landscape in 2016 (1) • Oral PrEP – Optimization of Truvada delivery – Increasing availability – US, Canada, France, South Africa, Kenya, Israel – 2nd generation regimens being evaluated • Maraviroc combinations • Tenofovir alafenamide – New molecules • EFdA (Merck) The PrEP Landscape in 2016 (2) • Injectable PrEP – Rilpivirine & Cabotegravir • Intravaginal rings – Phase 3 • Dapivirine – Phase 1/2 • Tenofovir • Tenofovir disoproxil fumarate • Vicriviroc + MK 2048 • Dapivirine + Levonorgestrol The PrEP Landscape in 2016 (3) • Rectal microbicides – Phase 2 • Reduced glycerin tenofovir gel – Phase 1 • Maraviroc • Dapivirine • Griffithsin • MIV-150 / Carageenan / Zinc • Implantable PrEP MTN Highlights from the Past Year The MTN Portfolio 2015 / 2016 Studies in Ongoing development: Completed studies MTN-026 studies MTN-030 MTN-015 MTN-031 MTN-017 MTN-016 MTN-032 MTN-020 MTN-023 MTN-033 MTN-024 MTN-027 MTN-028 MTN-034 MTN-029 MTN-035 MTN-036 MTN-037 Intravaginal Ring Studies • Dapivirine • Vicriviroc / MK-2048 – MTN-020 – MTN-027 & MTN-028 – MTN-023 • Dapivirine 200 mg – MTN-025 (HOPE) IVR – MTN-029 – MTN-036 / IPM 047 – MTN-031 – MTN-034 • Dapivirine / Levonorgestrel – MTN-030 / IPM 041 Rectal Microbicide Studies • Tenofovir gel – MTN-017 • Dapivirine gel – MTN-026 – MTN-033 – MTN-035 CHARM-02 Study (Hiruy H et al. -

Design and Evaluation of 5•²-O-Dicarboxylic And
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2014 DESIGN AND EVALUATION OF 5′-O-DICARBOXYLIC AND POLYARGININE FATTY ACYL DERIVATIVES OF ANTI-HIV NUCLEOSIDES Bhanu Priya Pemmaraju Venkata University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Pemmaraju Venkata, Bhanu Priya, "DESIGN AND EVALUATION OF 5′-O-DICARBOXYLIC AND POLYARGININE FATTY ACYL DERIVATIVES OF ANTI-HIV NUCLEOSIDES" (2014). Open Access Master's Theses. Paper 474. https://digitalcommons.uri.edu/theses/474 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. DESIGN AND EVALUATION OF 5′-O- DICARBOXYLIC AND POLYARGININE FATTY ACYL DERIVATIVES OF ANTI-HIV NUCLEOSIDES BY BHANU PRIYA, PEMMARAJU VENKATA A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE MASTER’S DEGREE IN BIOMEDICAL AND PHARMACEUTICAL SCIENCES UNIVERSITY OF RHODE ISLAND 2014 MASTER OF SCIENCE THESIS OF BHANU PRIYA, PEMMARAJU VENKATA APPROVED: Thesis Committee: Major Professor Keykavous Parang Roberta King Stephen Kogut Geoffrey D. Bothun Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT 2′,3′-Dideoxynucleoside (ddNs) analogs are the most widely used anti-HIV drugs in the market. Even though these drugs display very potent activities, they have a number of limitations when are used as therapeutic agents. The primary problem associated with ddNs is significant toxicity, such as neuropathy and bone marrow suppression. -

Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis
Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Centers for Disease Control and Prevention Office of Infectious Diseases National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination June 2013 This document is accessible online at http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Suggested citation: CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online]. 2013. Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Table of Contents Introduction 1 Methodology for Preparation of these Guidelines 2 The Role of Rifamycins in Tuberculosis Treatment 4 Managing Drug Interactions with Antivirals and Rifampin 5 Managing Drug Interactions with Antivirals and Rifabutin 9 Treatment of Latent TB Infection with Rifampin or Rifapentine 10 Treating Pregnant Women with Tuberculosis and HIV Co-infection 10 Treating Children with HIV-associated Tuberculosis 12 Co-treatment of Multidrug-resistant Tuberculosis and HIV 14 Limitations of these Guidelines 14 HIV-TB Drug Interaction Guideline Development Group 15 References 17 Table 1a. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in adults 21 Table 1b. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in children 22 Table 2a. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in adults 23 Table 2b. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in children 25 Table 3. Recommendations for co-administering antiretroviral drugs with RIFABUTIN in adults 26 ii Introduction Worldwide, tuberculosis is the most common serious opportunistic infection among people with HIV infection. -

Biochemical Engineering: Ta Da! a Way to Add Fluorine to Natural Products
RESEARCH HIGHLIGHTS IN BRIEF BIOCHEMICAL ENGINEERING Ta da! A way to add fluorine to natural products Adding fluorine to small molecules can improve their properties, such as preventing enzymatic breakdown and increasing membrane permeation, but it is hard to add fluorine to natural products. Walker et al. engineered polyketide synthase enzymes, which are normally involved in acetate-based biosynthesis, to incorporate fluoroacetate: the primary product of the only biological fluorination route. In Escherichia coli cells, this process was used as a building block to introduce fluorine substituents in a site-selective manner into polyketide-based natural product scaffolds. ORIGINAL RESEARCH PAPER Walker, M. C. et al. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science 341, 1089–1094 (2013) INFECTIOUS DISEASE Combating visceral leishmaniasis Visceral leishmaniasis is often fatal, yet current drugs are very toxic and incur resistance. Because Leishmania spp. require exogenous haem for growth, Guha et al. investigated whether haem acquisition could be a potential target. They found that the haemoglobin receptor (HbR) was conserved across several strains of Leishmania, and a HbR-specific antibody was detected in patients with visceral leishmaniasis. Immunization of mice and hamsters with HbR DNA completely protected against virulent Leishmania donovani infection and stimulated the production of protective cytokines as well as CD4+ and CD8+ T cells, which suggests that HbR is a target for vaccine development. ORIGINAL RESEARCH PAPER Guha, R. et al. Vaccination with Leishmania hemoglobin receptor-encoding DNA protects against visceral leishmanias. Sci. Transl. Med. 5, 202ra121 (2013) G PROTEIN-COUPLED RECEPTORS Crystal structures aid ligand mechanistics Two new papers on G protein-coupled receptor (GPCR) structure shed new light on how different ligands interact with their cognate GPCRs. -

Semen-Mediated Enhancement of HIV-1 Infection Markedly Impairs the Antiviral Efficacy of Microbicides
Institute of Molecular Virology Ulm University Medical Center Prof. Dr. Frank Kirchhoff Semen-mediated enhancement of HIV-1 infection markedly impairs the antiviral efficacy of microbicides Dissertation to obtain the Doctoral Degree of Human Biology (Dr. biol. hum.) at the Faculty of Medicine, University of Ulm presented by Onofrio Zirafi Heidenheim an der Brenz 2014 Present Dean: Prof. Dr. Thomas Wirth 1st reviewer: Prof. Dr. Jan Münch 2nd reviewer: Prof. Dr. Barbara Spellerberg Date of graduation: 5th December 2014 TABLE OF CONTENTS III TABLE OF CONTENTS ABBREVIATIONS ............................................................................................................. V 1 INTRODUCTION ....................................................................................................... 1 1.1 HIV LIFE CYCLE AND ANTIRETROVIRAL DRUGS .......................................................... 2 1.2 HIV TRANSMISSION ................................................................................................... 4 1.3 FACTORS IN SEMEN MODULATING HIV-1 INFECTION .................................................. 5 1.4 MECHANISM OF INFECTION ENHANCEMENT BY SEMINAL AMYLOIDS ........................... 6 1.5 COUNTERACTING AMYLOID-MEDIATED ENHANCEMENT OF HIV-1 INFECTION ............ 6 1.6 STRUCTURE-BASED PEPTIDE INHIBITORS OF AMYLOID FORMATION ............................. 7 1.7 MICROBICIDES ............................................................................................................ 8 1.8 SCIENTIFIC AIM ........................................................................................................ -

What Lessons Can We Learn from 20 Years of Chemokine T D Di ? Receptor
What lessons can we learn from 20 years of chemokine receptdtor drug discovery? John G. Cumming, PhD 5th RSC / SCI symposium on GPCRs in Medicinal Chemistry 15th-17th September 2014, Actelion, Allschwil, Basel, Switzerland Outline Background: chemokines and their receptors Chemokine receptor drug discovery and development Emerging opportunities for chemokine drug discovery Conclusions and learning Chemokines and chemokine receptors CXC(α) • Chemokines (chemoattractant cytokines) are 70-120 aa proteins • 44 chemokines in 4 major families and 22 chemokine receptors in human genome • ‘Cell positioning system’ in the body • Many receptors bind multiple ligands • Many ligands bind multiple receptors Chemotaxis Human monocytes + CCL2 (red) Volpe et al. PLoS ONE 2012, 7(5), e37208 CCR2 antagonists inhibit chemotaxis and infiltration Vasculature CCL2 release Spinal or Peripheral Tissue Recruited monocyte Site of CCL2 release CCR2 antagonists inhibit chemotaxis and infiltration CCR2 antagonist Circulating monocyte CCL2 release CCL2 release from peripheral injury site or central PAF terminals Role of chemokine system in pathophysiology • Potential role in inflammatory and autoimmune diseases: Multiple sclerosis, Rheumatoid arthritis, COPD, allergic asthma, IBD, psoriasis - Expression levels of chemokines and receptors in relevant tissues and organs of patients and animal disease models - Mouse knockout ppyphenotype in disease models • Established role in HIV infection Katschke et al., 2001 Arthritis Rheum, 44, 1022 - CCR5 and CXCR4 act as HIV-1 -
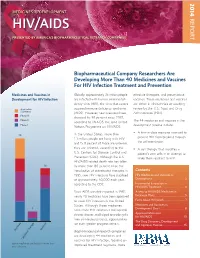
14-258 Phrma HIV/AIDS2014 0819.Indd
2014 MEDICINES IN DEVELOPMENT REPORT HIV/AIDS PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Company Researchers Are Developing More Than 40 Medicines and Vaccines For HIV Infection Treatment and Prevention Medicines and Vaccines in Globally, approximately 35 million people effective therapies, and preventative Development for HIV Infection are infected with human immunodefi - vaccines. These medicines and vaccines ciency virus (HIV), the virus that causes are either in clinical trials or awaiting Application acquired immune defi ciency syndrome review by the U.S. Food and Drug Submitted (AIDS). However, new infections have Administration (FDA). Phase III dropped by 38 percent since 2001, Phase II The 44 medicines and vaccines in the according to UNAIDS, the Joint United Phase I development pipeline include: Nations Programme on HIV/AIDS. • A fi rst-in-class medicine intended to In the United States, more than 25 prevent HIV from breaking through 1.1 million people are living with HIV the cell membrane. and 15.8 percent of those are unaware they are infected, according to the • A cell therapy that modifi es a U.S. Centers for Disease Control and patient’s own cells in an attempt to Prevention (CDC). Although the U.S. make them resistant to HIV. HIV/AIDS-related death rate has fallen 16 by more than 80 percent since the introduction of antiretroviral therapies in Contents 1995, new HIV infections have stabilized HIV Medicines and Vaccines in at approximately 50,000 each year, Development ......................................2 according to the CDC. Incremental Innovation in HIV/AIDS Treatment .......................... 4 Since AIDS was fi rst reported in 1981, Access to HIV/AIDS Medicines in nearly 40 medicines have been approved Exchange Plans ...................................5 to treat HIV infection in the United Facts About HIV/AIDS ........................7 States. -

A Novel Small Molecule CCR5 Inhibitor Active Against R5-Tropic HIV-1S
www.nature.com/scientificreports OPEN Activity and structural analysis of GRL-117C: a novel small molecule CCR5 inhibitor active against R5- Received: 24 September 2018 Accepted: 1 March 2019 tropic HIV-1s Published: xx xx xxxx Hirotomo Nakata1,2, Kenji Maeda 3, Debananda Das 1, Simon B. Chang1, Kouki Matsuda3, Kalapala Venkateswara Rao4, Shigeyoshi Harada5, Kazuhisa Yoshimura5, Arun K. Ghosh4 & Hiroaki Mitsuya1,2,3 CCR5 is a member of the G-protein coupled receptor family that serves as an essential co-receptor for cellular entry of R5-tropic HIV-1, and is a validated target for therapeutics against HIV-1 infections. In the present study, we designed and synthesized a series of novel small CCR5 inhibitors and evaluated their antiviral activity. GRL-117C inhibited the replication of wild-type R5-HIV-1 with a sub-nanomolar IC50 value. These derivatives retained activity against vicriviroc-resistant HIV-1s, but did not show activity against maraviroc (MVC)-resistant HIV-1. Structural modeling indicated that the binding of compounds to CCR5 occurs in the hydrophobic cavity of CCR5 under the second extracellular loop, and amino acids critical for their binding were almost similar with those of MVC, which explains viral cross- resistance with MVC. On the other hand, one derivative, GRL-10018C, less potent against HIV-1, but more potent in inhibiting CC-chemokine binding, occupied the upper region of the binding cavity with its bis-THF moiety, presumably causing greater steric hindrance with CC-chemokines. Recent studies have shown additional unique features of certain CCR5 inhibitors such as immunomodulating properties and HIV-1 latency reversal properties, and thus, continuous eforts in developing new CCR5 inhibitors with unique binding profles is necessary.