The Clean Microscope (Zeiss)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

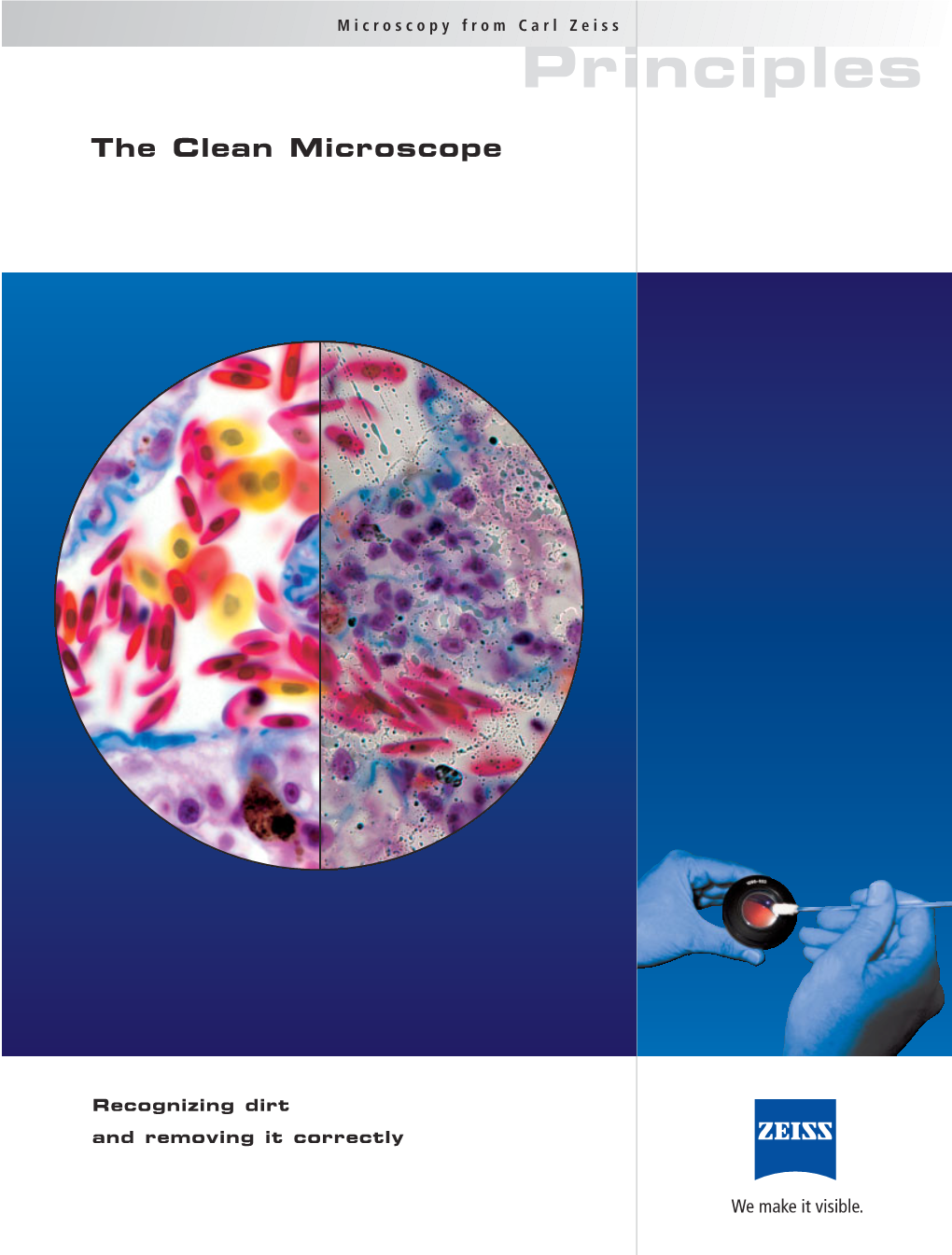
Resolving Power
The 5 I’s of Culturing Microbes • Inoculation • Isolation • Incubation • Inspection • Identification 8/18/12 MDufilho 1 Table 4.1 Metric Units of Length 8/18/12 MDufilho 2 Microscopy • General Principles of Microscopy – Wavelength of radiation – Magnification – Resolution – Contrast 8/18/12 MDufilho 3 Figure 4.1 The electromagnetic spectrum 400 nm 700 nm Visible light X UV Infra- Micro- Radio waves and Gamma rays rays light red wave Television Increasing wavelength 10–12m 10–8m 10–4m 100m 103m Crest One wavelength Trough Increasing resolving power 8/18/12 MDufilho 4 Figure 4.2 Light refraction and image magnification by a convex glass lens-overview Light Air Glass Focal point Specimen Convex Inverted, lens reversed, and enlarged 8/18/12 MDufilho image 5 Principles of Light Microscopy • Magnification occurs in two phases – – The objective lens forms the magnified real image – The real image is projected to the ocular where it is magnified again to form the virtual image • Total magnification of the final image is a product of the separate magnifying powers of the two lenses power of objective x power of ocular = total magnification 8/18/12 MDufilho 6 8/18/12 MDufilho 7 Resolution Resolution defines the capacity to distinguish or separate two adjacent objects – resolving power – Function of wavelength of light that forms the image along with characteristics of objectives • Visible light wavelength is 400 nm–750 nm • Numerical aperture of lens ranges from 0.1 to 1.25 • Oil immersion lens requires the use of oil to prevent refractive loss -

Small Wonder Acetone Vaporizer
Acetone Vaporizers and Clearing Supplies: 84-85 Microscope Slides & Cover Glass: 86-87 Sample Collection and Storage Bags: 88 Respirator Fit Test Kits: 89-91 Small Wonder Acetone Vaporizer The Wonder Makers Small Wonder Acetone Vaporizer was developed as an economical and quick way to clear asbestos PCM slides. Specifically designed to meet NIOSH 7400, 7402, or ORM requirements for preparation of PCM samples. Features: ■ Heat control and thermometer built into vaporizer unit ■ Padded carrying case protects equipment and supplies ■ Detailed instruction manual ■ Enough supplies for the analysis of 1/2 gross of slides ■ Lightweight but solidly built ■ Designed in strict compliance with the NIOSH 7400, 7402, or ORM requirements for preparation of microscope slides for PCM analysis Specifications 4-7/8” L x 4-1/2” W x 2-3/4” H (Small Wonder vaporizer) DIMENSIONS 13” L x 11” W x 4” H (Complete Analysis Kit) Plastic case, ABS plastic cover on vaporizer, clear MATERIALS plastic bottles, metal and plastic tools. 2-1/2 lbs. (Small Wonder vaporizer) WEIGHT 8 lbs. (Complete Analysis Kit) Black vaporizer cover with FINISH gray cord. Case color may vary. Complete Kit Shown Includes everything needed to clear slides for PCM analysis PRODUCT DESCRIPTION Small Wonder Analysis Kit WM-42 Includes acetone vaporizer, 1 box microscope slides, 1/2oz Triacetin, 2oz Acetone, 1 microdropper, 1 syringe, 1 scalpel, tweezers, padded carrying case and instruction manual. WM-44 Acetone reagent, 2oz, 99% pure WM-45 Triacetin reagent, 1/2oz, 99% pure WM-47 1cc Syringe WM-48 Rounded Edge Scalpel WM-49 Microdropper 81 The Professionals Choice for Air Sampling Equipment Perm-O-Fix Acetone Vaporizer A simple, easy to use, actone vaporizer for use in preparing asbestos PCM samples as per NIOSH 7400. -

Using Microscopes
Using Microscopes Grade Level: 6, 7, 8, 9, 10 Duration: 30-45 minutes Classification: Classroom Subject(s): Biology, Microbiology Categories (STEM): Science, Technology Keywords: Staining, Microscopes, Microbes Introduction ● Summary: Students will learn how to properly use microscopes and view different cells and organisms. ● Description: Microscopes are often used to view different types of organisms, cells, and organelles. Microscopes can be used to visualize changes in cell structure and identify unknown organisms. Students will learn all the parts of microscopes and how to use and adjust them. Another important step of using microscopes is to learn how to properly prepare slides and wet mounts. Different organisms, cells, and organelles will be viewed under a microscope. In case of school policy limitations, note that student role models are required to cut vegetables at schools before the visit starts. *Note - School is responsible for providing microscopes for this activity. Online Resources: https://www.youtube.com/watch?v=SUo2fHZaZCU\ Vocabulary Organisms: an individual animal, plant, or single-celled life form Cell: smallest structural unit of an organism Organelle: organized structures (compartments) within a living cell Nucleus: dense organelle (generally in the middle of a cell) that contains genetic information Wet Mount: microscope slide holding a specimen suspended in a drop of liquid Materials Materials Quantity Reusable? Nail Polish 1 red per classroom Yes Microscopes (provided by school) TBD by school Yes Dirty -

Microscope Slide-Making Kit
Microscope Slide-Making Kit 665 Carbon Street, Billings, MT 59102 Phone: 800.860.6272 Fax: 888.860.2344 www.homesciencetools.com Copyright 2005 by Home Training Tools, Ltd. All rights reserved. Viewing Slides Scan a slide at low power (usually 40X) to get an overview of the specimen. Center the part of the specimen you want to view at higher power. Adjust your lighting until the slide specimen has clear, sharp contrast. Then switch to medium power (usually 100X) and refocus to observe tissue and cell variations. Repeat at high power (usually 400X). Making Your Own Slides Whole Mounts: Whole mounts are made by placing small objects or specimens whole on a blank slide and then covering them with a coverslip. If your specimen is thick, a concavity slide will work better. You can make whole mounts of many things. Here are some ideas: Colored thread Feather (piece) Thin plant leaf Print (letters) Cloth fibers Hair strand Small insects Dust Algae Pond water Thin paper Insect parts Whole mount specimens must be thin enough to allow light to pass through them. Do not use large, hard objects like rocks, as they can break your slides and microscope lenses. Sections: Section mounts are made by slicing a very thin section of specimen. A cross section is made by slicing across the width or diameter of the specimen. A longitudinal section is made by slicing across the length of the specimen. It is difficult to make sections thin enough without an instrument called a microtome. You can make a simple microtome with a thread spool, a 1/4” diameter bolt at least 2” long, and a 1/4” nut. -

An Antique Microscope Slide Brings the Thrill of Discovery Into a Contemporary Biology Classroom
ARTICLE An Antique Microscope Slide Brings the Thrill of Discovery into a Contemporary Biology Classroom FRANK REISER ABSTRACT that few others share my interests), I add them to my growing collection. The discovery of a Victorian-era microscope slide titled “Grouped Flower Seeds” began When I found skillfully prepared Victorian-era microscope slides listed an investigation into the scientific and historical background of the antique slide to under the category “Folk Art” in an antique dealer’s online catalog, it led develop its usefulness as a multidisciplinary tool for PowerPoint presentations usable in not only to my acquiring them but also to the beginning of a journey contemporary biology classrooms, particularly large-enrollment sections. The resultant involving Internet and library research, trips to herbaria, field collecting presentation was intended to engage students in discussing historical and contempo- excursions, and, finally, to the development of a PowerPoint lecture for rary biology education, as well as some of the intricacies of seed biology. Comparisons between the usefulness and scope of various seed identification resources, both online community college biology students. The slide that triggered the snow- and in print, were made. balling project is titled “Grouped Flower Seeds” (Figure 1). Having spent most of my life as a biology educator, I view most of Key Words: Grouped Flower Seeds; Watson and Sons; antique microscope slide; what I encounter in life from a science-oriented perspective. Finding pre- online seed identification resources; seed identification manuals; seed biology; pared microscope slides described as “folk art” sharply conflicted with large-enrollment lecture. my sense of the correct order of disciplines – particularly for items that I would reverently classify as the tools of scientists. -

Microscopic Exam of Urine Sediment 1
Name:___________________________ Seat # ______ Do not write in pencil (use pen). Do not use Liquid Paper (draw one line through error). Date: ____/____/____ Periods_______________ Please staple all work. Routine Urinalysis with Microscopic Lab Title 4 Lab Number Purpose: The purpose of this lab is to learn how to identify sediment found in urine and learn the clinical significance of urine sediment. Materials: 1. cup to collect urine sample 6. (2) coverslips 11. Laboratory Coat 2. centrifuge 7. test tube rack 12. Disinfecting wipes 3. plastic pipette 8. Microscope 13. Biohazard containers 4. centrifuge tube 9. Sedi-stain 14. Refactometer 5. (1) microscope slide 10. Working Mat (paper towel) 15. Beaker of water Procedure(s): See student handout and pictures to help aid in your procedure. Microscopic Exam of Urine Sediment 1. Collect a fresh urine sample. Obtain a centrifuge tube and put your seat number at the very top of tube. 2. Pour or pipette 12 ml of urine into the centrifuge tube. 3. Centrifuge tube for 5 minutes. Centrifuge Procedure: a. After pouring 12 ml of urine in tube, put in centrifuge and balance your tube with 12 ml of fluid in the same type of tube (pair up with another student). b. Position tubes directly across from each other. c. The teacher will demonstrate how to close and lock lid, and set timer and speed. d. Centrifuge will stop automatically. e. Make sure the centrifuge comes to a complete stop before opening. (Remember the movie OutBreak) ***While your urine is spinning, review how to use the microscope properly (see lesson in lab manual). -

Ideal Conditions for Urine Sample Handling, and Potential in Vitro Artifacts Associated with Urine Storage
Urinalysis Made Easy: The Complete Urinalysis with Images from a Fully Automated Analyzer A. Rick Alleman, DVM, PhD, DABVP, DACVP Lighthouse Veterinary Consultants, LLC Gainesville, FL Ideal conditions for urine sample handling, and potential in vitro artifacts associated with urine storage 1) Potential artifacts associated with refrigeration: a) In vitro crystal formation (especially, calcium oxalate dihydrate) that increases with the duration of storage i) When clinically significant crystalluria is suspected, it is best to confirm the finding with a freshly collected urine sample that has not been refrigerated and which is analyzed within 60 minutes of collection b) A cold urine sample may inhibit enzymatic reactions in the dipstick (e.g. glucose), leading to falsely decreased results. c) The specific gravity of cold urine may be falsely increased, because cold urine is denser than room temperature urine. 2) Potential artifacts associated with prolonged storage at room temperature, and their effects: a) Bacterial overgrowth can cause: i) Increased urine turbidity ii) Altered pH (1) Increased pH, if urease-producing bacteria are present (2) Decreased pH, if bacteria use glucose to form acidic metabolites iii) Decreased concentration of chemicals that may be metabolized by bacteria (e.g. glucose, ketones) iv) Increased number of bacteria in urine sediment v) Altered urine culture results b) Increased urine pH, which may occur due to loss of carbon dioxide or bacterial overgrowth, can cause: i) False positive dipstick protein reaction ii) Degeneration of cells and casts iii) Alter the type and amount of crystals present 3) Other potential artifacts: a) Evaporative loss of volatile substances (e.g. -

Microbial Discovery Activity Pond Scum – Page 2
MMMiiicccrrrooobbbiiiaaalll DDDiiissscccooovvveeerrryyy AAAccctttiiivvviiitttyyy PPPooonnnddd SSScccuuummm::: IIInnnvvveeessstttiiigggaaatttiiinnnggg MMMiiicccrrroooooogggrraaannniiisssmmmsss Author Mark Gallo, Ph.D. Associate Professor of Biology Niagara University, NY 14109 [email protected] Contributor George A. Jacob, Ph.D. St. Xavier High School Cincinnati, OH 45224 [email protected] Intended Audience K-4 X 5-8 X 9-12 X Activity Specifications Classroom setting X Requires special equipment X Uses hands-on manipulatives X Requires mathematical skills Can be performed individually X Requires group work Requires more than one (45 min) class period X Appropriate for special needs student X American Society for Microbiology Education Department 1752 N Street, NW Washington, DC 20036 [email protected] IIInnntttrrroooddduuuccctttiiiooonnn Description In this exercise, students discover a diversity of microorganisms living in a small drop of pond water. Abstract Little else can stimulate a student’s interest in biology like a drop of pond water teaming with invisible life viewed with a microscope. This activity describes two means of observing pond water other than the traditional hanging drop or temporary wet mount slide preparation. Core Themes Addressed Microorganisms and the Environment Keywords Aquatic, Environment, Protozoa, Biofilm, Water quality, Classification Learning Objectives At completion of this activity, learner will be able to: • appreciate (acknowledge) the previously unseen life in pond water. • prepare a biofilm slide. • draw and describe a microorganism. National Science Education Standards Addressed Unifying Concepts and Processes – This activity incorporates evidence of microscopic life. Standard 1: Science as Inquiry – In completion of this activity student performs directed, inquiry based observations of microorganisms. Standard 3: Life science - In completion of this activity student will have had a chance to view living organisms from an environment and determine the characteristics of the microorganisms. -

Practical Tips for Two-Photon Microscopy
Appendix 1 Practical Tips for Two-Photon Microscopy Mark B. Cannell, Angus McMorland, and Christian Soeller INTRODUCTION blue and green diode lasers. To provide an alignment beam to which the external laser can be aligned, light from this reference As is clear from a number of the chapters in this volume, 2-photon laser needs to be bounced back through the microscope optical microscopy offers many advantages, especially for living-cell train and out through the external coupling port: studies of thick specimens such as brain slices and embryos. CAUTION: Before you switch on the reference laser in this However, these advantages must be balanced against the fact that configuration make sure that all PMTs are protected and/or commercial multiphoton instrumentation is much more costly than turned off. the equipment used for confocal or widefield/deconvolution. Given Place a front-surface mirror on the stage of the microscope and these two facts, it is not surprising that, to an extent much greater focus onto the reflective surface using an air objective for conve- than is true of confocal, many researchers have decided to add a nience (at sharp focus, you should be able to see scratches or other femtosecond (fs) pulsed near-IR laser to a scanner and a micro- mirror defects through the eyepieces). The idea of this method is scope to make their own system (Soeller and Cannell, 1996; Tsai to cause the reference laser beam to bounce back through the et al., 2002; Potter, 2005). Even those who purchase a commercial optical train and emerge from the other laser port. -

Innovative Methods of Archiving, Presentation and Providing Access to Histological Sections
ADVANCES IN CELL BIOLOGY VOL. 3, ISSUE 3/2011 (41–54) INNOVATIVE METHODS OF ARCHIVING, PRESENTATION AND PROVIDING ACCESS TO HISTOLOGICAL SECTIONS Krystyna FILIPIAK, Agnieszka MALIŃSKA, Dariusz KRUPA, Maciej ZABEL Department of Histology and Embryology, Poznań University of Medical Sciences, Poland DOI: 10.2478/v10052-011-0003-4 Summary: The dynamic development of technical sciences and informatics makes now possible acquisition of microscopic images of histological sections, not only using digital cameras, but also through specialized devices called scanners. The digitalized images stored in a computer storage device are called virtual slides and, together with special software, are known as virtual microscopy. The virtual slides can be analyzed on a computer screen by panoramic viewing or using a detailed image examination at higher magnification. In many research and education institutions in both the U.S. and Europe, the virtual microscopy is used for teaching and training purposes. In the academic year of 2009/10, Department of Histology and Embryology, University of Medical Sciences in Poznan, as one of the first in Poland, has created a virtual database for educational purposes. This database created by archiving the traditional images of histological slides in the form of digital images. So far, more than 130 virtual slides have been acquired and catalogued in 24 thematic folders, available for medical students participating in histology, embryology and cell biology courses. Telepathology is the second branch which uses virtual microscopy. Virtual microscope allows to discuss and resolve medical/diagnostic problems with the use of telecommunication systems and information technology. The existing internet platforms offer access to virtual microscopes and virtual slides. -

STAINING TECHNIQUES Staining Is an Auxiliary Technique Used in Microscopy to Enhance Contrast in the Microscopic Image
STAINING TECHNIQUES Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Stains or dyes are used in biology and medicine to highlight structures in biological tissues for viewing with microscope. Cell staining is a technique that can be used to better visualize cells and cell components under a microscope. Using different stains, it is possible to stain preferentially certain cell components, such as a nucleus or a cell wall, or the entire cell. Most stains can be used on fixed, or non-living cells, while only some can be used on living cells; some stains can be used on either living or non-living cells. In biochemistry, staining involves adding a class specific (DNA, lipids, proteins or carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescence tagging can serve similar purposes Purposes of Staining The most basic reason that cells are stained is to enhance visualization of the cell or certain cellular components under a microscope. Cells may also be stained to highlight metabolic processes or to differentiate between live and dead cells in a sample. Cells may also be enumerated by staining cells to determine biomass in an environment of interest. Stains may be used to define and examine bulk tissues (e.g. muscle fibers or connective tissues), cell populations (different blood cells) or organelles within individual cells. Biological staining is also used to mark cells in flow cytometry, flag proteins or nucleic acids on gel electrophoresis Staining is not limited to biological materials, it can also be used to study the morphology (form) of other materials e.g. -

Extension of Solid Immersion Lens Technology to Super-Resolution Raman Microscopy
Nanospectroscopy 2014; 1: 1–11 Research Article Open Access Edwin Ostertag*, Anita Lorenz, Karsten Rebner, Rudolf W. Kessler, Alfred J. Meixner Extension of solid immersion lens technology to super-resolution Raman microscopy Abstract: Scanning Near-Field Optical Microscopy 1 The near-field accessed with solid (SNOM) has developed during recent decades into a valuable tool to optically image the surface topology of immersion lens technology materials with super-resolution. With aperture-based The main reason for using solid immersion lenses is SNOM systems, the resolution scales with the size of the their capability to enhance the spatial resolution. Solids aperture, but also limits the sensitivity of the detection have higher refractive indices than air. When used as the and thus the application for spectroscopic techniques immersion medium, light travels more slowly and the like Raman SNOM. In this paper we report the extension wavelength is shorter in the optically denser medium of solid immersion lens (SIL) technology to Raman SNOM. which results in an improvement in resolution. Mansfield The hemispherical SIL with a tip on the bottom acts as and Kino were the first to recommend the SIL for an apertureless dielectric nanoprobe for simultaneously increasing the spatial resolution of the optical microscope acquiring topographic and spectroscopic information. [1]. A SIL is a spherical lens (diameter 2r, refractive index The SIL is placed between the sample and the microscope n ) which is polished to a thickness of r (hemisphere objective of a confocal Raman microscope. The lateral SIL type) or (1+1/n )r (supersphere or Weierstrass type) [2,3].