Antepartum Haemorrhage of Unknown Origin and Maternal Cigarette Smoking Beyond the first Trimester
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Clinical an Urgent Care Approach to Complications and Conditions of Pregnancy Part 2
Clinical An Urgent Care Approach to Complications and Conditions of Pregnancy Part 2 Urgent message: From pregnancy confirmation to the evaluation of bleeding, urgent care centers are often the initial location for management of obstetric-related issues. Careful use of evidence-based guidelines is the key to successful outcomes. DAVID N. JACKSON, MD, FACOG and PETAR PLANINIC, MD, FACOG Introduction rgent care providers are called upon to manage a Uvariety of complaints in pregnancy. Some conditions can be managed at the urgent care center whereas others require stabilization and transport to a center with expert obstetrical capabilities. In all situations, practitioners should consider that a gestational age of fetal viability (many centers now use 23 to 24 weeks) is best served with referral for continuous fetal monitor- ing if there is bleeding, trauma, significant hypertension, relative hypoxemia (O2 saturation less than 95% for pregnant women), or contractions. Part 2 of this two- part series will discuss: Ⅲ Bleeding in pregnancy Ⅲ Ectopic gestation Ⅲ Trauma and pregnancy Ⅲ Acute abdominal pain in pregnancy Dr. Jackson is Professor of Maternal-Fetal Medicine at the University of Nevada, School of Medicine, Las Vegas, Nevada. Dr. Planinic is Assistant Professor of Obstetrics and Gynecology at the University of Nevada, School of Medicine, Las Vegas, Nevada. © gettyimages.com www.jucm.com JUCM The Journal of Urgent Care Medicine | September 2013 9 AN URGENT CARE APPROACH TO COMPLICATIONS AND CONDITIONS OF PREGNANCY Figure 1. Bleeding endocervical polyp with Evaluation of vaginal bleeding should follow a sys- inflammation tematic process. History of last menses and sexual activ- ity determines the possibility of pregnancy. -

Clinical Relevance of Bleeding Per Vaginam in Early Pregnancy (Before 26 Weeks Gestation) in Patients Recruited from 28Weeks to Delivery
Clinical Relevance of Bleeding Per Vaginam in Early Pregnancy (Before 26 Weeks Gestation) in Patients Recruited from 28weeks to Delivery *Chukwunyere A, *Enaruna N Correspondence Dr. N, Enaruna *Department of Obstetrics and Gynaecology, Department of Obstetrics and Gynaecology, University of Benin Teaching Hospital, Benin University of Benin Teaching Hospital City, Nigeria. Benin City, Edo State Nigeria. E-mail: [email protected] Citation: Chukwunyere A, Enaruna N (2020). Clinical relevance of bleeding per vaginam in early pregnancy (before 26 weeks gestation) in patients recruited from 28weeks to delivery. Nig J Med Dent Educ; 2(2):c28-c31. INTRODUCTION imaging studies are then used for confirmation Bleeding per vaginum in pregnancy is a common (Oguntoyinbo, 2011). presentation in obstetrics. Incidence in literature The objective of this study was to demonstrate ranges from 12% to as high as 40% (Olugbenga, clinical relevance of bleeding per vaginam in early 2019). It can occur in all stages of pregnancy but pregnancy (before 26 weeks gestation). commoner in early pregnancy and has been reported to affect 20% to 30% of pregnancies (Kalyani 2015). MATERIALS AND METHODS The aetiology and source is most times always Women with bleeding per vaginum in early maternal, rather than fetal. Bleeding can result from pregnancy were approached and those who agreed disruption of blood vessels in the decidua or from to participate and gave informed consent were discrete cervical or vaginal lesions (Gupta, 2016; recruited. Information regarding the experience of Tiparse, 2017). Common aetiology includes bleeding in early pregnancy documented and threatened miscarriage, miscarriage, ectopic relevant data on sociodemographic characteristics, gestation and molar gestation. -

Ectopic Pregnancy PPT B&W
Early pregnancy bleeding Ectopic pregnancy Dr. Kakali Saha MBBS, FCPS, MS (Obs & Gynae) Associate Professor Medical College for Women & Hospital Ectopic pregnancy • Definition : An ectopic pregnancy is one in which the fertilised ovum becomes implanted in a site other than the normal uterine cavity. • Extrauterine pregnancy -but rudimentary horn of a bicornuate uterus. • It is the consequence of an abnormal implantation of the blastocyst. Incedence • Worldwide 3-4% of all pregnancy. • In USA 2% • Some study 16 in 1000. Past 20 years incidence risen ✦ After one ectopic - there is a7-13 fold increase risk of subsequent ectopic ✦ Subsequent intrauterine preg —50-80% ✦Tubal preg 10-25% ✦Infertile — remaining patient Sites of ectopic pregnancy According to frequency • Fallopian tubes 95-98% (At fimbriated end 17%, Ampulla-55%,Isthmus 25% interstitial 3%) • Uterine cornu 2-2.5% • Ovary, Cervix & abdominal cavity <1% • Right side is more common than left. Risk factors • PID (pelvic inflammatory diseases —6 fold increases risk • Use of IUCD —3-5% increased risks • Smoking 2.5% increased risks • ART 3-5% increased risks • Tubal damage • Tubal surgery 5.8% • Salpingitis isthmica nodes 3.5% increased risks • Prior ectopic pregnancy cont. Risk factor • Age 3 fold increased risks in 35-44 years compared to 18 -24 yrs • Non white race 1.5 fold increased risks • Endometriosis 1.5 increased risks • Developmental errors • Overdevelopment of ovum & external migration . Aetiology • Tubal damage or altered motility results improper transport of blastocyst • Most common cause is acute salpingitis 50% • In 40% no risk factors apparent • Salpingitis causes peritubal adhesion , lumen occlusion , intratubal adhesion diverticula & disturbed tubal function. -
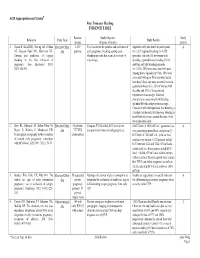
ACR Appropriateness Criteria® First Trimester Bleeding EVIDENCE TABLE
ACR Appropriateness Criteria® First Trimester Bleeding EVIDENCE TABLE Patients/ Study Objective Study Reference Study Type Study Results Events (Purpose of Study) Quality 1. Hasan R, Baird DD, Herring AH, Olshan Review/Other 4,539 To characterize the patterns and predictors of Approximately one-fourth of participants 4 AF, Jonsson Funk ML, Hartmann KE. -Dx patients early pregnancy bleeding, setting aside (n=1,207) reported bleeding (n=1,656 Patterns and predictors of vaginal bleeding episodes that occur at the time of episodes), but only 8% of women with bleeding in the first trimester of miscarriage. bleeding, reported heavy bleeding. Of the pregnancy. Ann Epidemiol 2010; spotting and light bleeding episodes 20(7):524-531. (n=1,555), 28% were associated with pain. Among heavy episodes (n=100), 54% were associated with pain. Most episodes lasted less than 3 days, and most occurred between gestational weeks 5–8. 12% of women with bleeding and 13% of those without experienced miscarriage. Maternal characteristics associated with bleeding included fibroids and prior miscarriage. Consistent with the hypothesis that bleeding is a marker for placental dysfunction, bleeding is most likely to be seen around the time of the luteal-placental shift. 2. Bree RL, Edwards M, Bohm-Velez M, Review/Other 53 patients; Compare TVUS with -hCG level in the -hCG level of 1000 mIU/ml - gestational sac 4 Beyler S, Roberts J, Mendelson EB. -Dx 75 TVUS evaluation of embryo in early pregnancy. was seen sonographically in each patient. - Transvaginal sonography in the evaluation examinations hCG level of 7200 mIU/ml - yolk sac was of normal early pregnancy: correlation seen in every patient. -

First Trimester Vaginal Bleeding EVIDENCE TABLE
ACR Appropriateness Criteria® First Trimester Vaginal Bleeding EVIDENCE TABLE Patients/ Study Objective Study Reference Study Type Study Results Events (Purpose of Study) Quality 1. Hasan R, Baird DD, Herring AH, Olshan Review/Other- 4,539 To characterize the patterns and predictors of Approximately one-fourth of participants 4 AF, Jonsson Funk ML, Hartmann KE. Dx patients early pregnancy bleeding, setting aside (n=1,207) reported bleeding (n=1,656 Patterns and predictors of vaginal bleeding episodes that occur at the time of episodes), but only 8% of women with bleeding in the first trimester of miscarriage. bleeding, reported heavy bleeding. Of the pregnancy. Ann Epidemiol. spotting and light bleeding episodes 2010;20(7):524-531. (n=1,555), 28% were associated with pain. Among heavy episodes (n=100), 54% were associated with pain. Most episodes lasted less than 3 days, and most occurred between gestational weeks 5–8. 12% of women with bleeding and 13% of those without experienced miscarriage. Maternal characteristics associated with bleeding included fibroids and prior miscarriage. Consistent with the hypothesis that bleeding is a marker for placental dysfunction, bleeding is most likely to be seen around the time of the luteal-placental shift. 2. Barnhart KT. Early pregnancy failure: Review/Other- N/A To review some of the pitfalls of the modern No results stated in abstract. 4 beware of the pitfalls of modern Dx management of early pregnancy failure and management. Fertil Steril. introduce a series of articles on the subject. 2012;98(5):1061-1065. 3. Bree RL, Edwards M, Bohm-Velez M, Review/Other- 53 patients; Compare TVUS with b-hCG level in the b-hCG level of 1000 mIU/ml - gestational sac 4 Beyler S, Roberts J, Mendelson EB. -

Progesterone to Prevent Miscarriage in Women with Early Pregnancy Bleeding: the PRISM RCT
Journals Library Health Technology Assessment Volume 24 • Issue 33 • June 2020 ISSN 1366-5278 Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT Arri Coomarasamy, Hoda M Harb, Adam J Devall, Versha Cheed, Tracy E Roberts, Ilias Goranitis, Chidubem B Ogwulu, Helen M Williams, Ioannis D Gallos, Abey Eapen, Jane P Daniels, Amna Ahmed, Ruth Bender-Atik, Kalsang Bhatia, Cecilia Bottomley, Jane Brewin, Meenakshi Choudhary, Fiona Crosfill, Shilpa Deb, W Colin Duncan, Andrew Ewer, Kim Hinshaw, Thomas Holland, Feras Izzat, Jemma Johns, Mary-Ann Lumsden, Padma Manda, Jane E Norman, Natalie Nunes, Caroline E Overton, Kathiuska Kriedt, Siobhan Quenby, Sandhya Rao, Jackie Ross, Anupama Shahid, Martyn Underwood, Nirmala Vaithilingham, Linda Watkins, Catherine Wykes, Andrew W Horne, Davor Jurkovic and Lee J Middleton DOI 10.3310/hta24330 Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT Arri Coomarasamyo ,1* Hoda M Harbo ,1 Adam J Devallo ,1 Versha Cheedo ,2 Tracy E Robertso ,2 Ilias Goranitiso ,3 Chidubem B Ogwuluo ,2 Helen M Williamso ,1 Ioannis D Galloso ,1 Abey Eapeno ,4 Jane P Danielso ,5 Amna Ahmedo ,6 Ruth Bender-Atiko ,7 Kalsang Bhatiao ,8 Cecilia Bottomleyo ,9 Jane Brewino ,10 Meenakshi Choudharyo ,11 Fiona Crosfillo ,12 Shilpa Debo ,13 W Colin Duncano ,14 Andrew Ewero ,1 Kim Hinshawo ,6 Thomas Hollando ,15 Feras Izzato ,16 Jemma Johnso ,17 Mary-Ann Lumsdeno ,18 Padma Manda,19 Jane E Normano ,14 Natalie Nuneso ,20 Caroline E Overtono ,21 Kathiuska Kriedto ,9 Siobhan -

Ultrasonography Findings in First Trimester Vaginal Bleeding
Nep J Obstet Gynecol. 2020;15(31):106-108 Original Article Ultrasonography findings in first trimester vaginal bleeding Rani Jha, Ankur Shah, Shailesh Kumar Jha Mithila Hospital Private Limited, Janakpur, Nepal Received: May 24, 2020 Accepted: October 16, 2020 ABSTRACT Aims: To determine the causes of first trimester vaginal bleeding using Ultrasonography. Methods: This is a hospital based cross sectional study conducted from July to December 2019. Ultrasonogram scan was done for the 200 women within 12 weeks of pregnancy with a positive pregnancy test and vaginal bleeding in in out-patient set up. Results: Majority were under 30 years of age and 45.5% diagnosed as threatened Abortion. More than half were non-viable pregnancy. Conclusions: Ultrasound examination is the essential diagnostic and confirmatory tool to diagnose early pregnancy bleeding in clinical set up. Keywords: Clinical examination, first trimester bleeding, ultrasonography Citation: Jha R, Shah A, Jha SK. Ultrasonography findings in first trimester vaginal bleeding. Nep J Obstet Gynecol. 2020;15(31):106– 108. DOI: https://doi.org/10.3126/njog.v15i2.32919 INTRODUCTION trimester bleeding can be done from the diagnostic and prognostic point of view. The first trimester of pregnancy includes first 12 weeks of pregnancy. First trimester vaginal bleeding The causes of early pregnancy bleeding can is an alarming and worrisome condition both for be: Implantation bleeding, threatened abortion, patient and the clinician. The incidence of bleeding blighted ovum, missed abortion, inevitable in first trimester is 7-24%1-5 and this wide variation abortion, incomplete abortion , complete abortion, can be due to different type of study design. -

Management of Vaginal Bleeding Presenting to the Accident and Emergency Department
130 3 Accid Emerg Med 1999;16:130-135 CLINICAL MANAGEMENT J Accid Emerg Med: first published as 10.1136/emj.16.2.130 on 1 March 1999. Downloaded from Management of vaginal bleeding presenting to the accident and emergency department Karen Buckingham, Alison Fawdry, Diana Fothergill Vaginal bleeding is a common presenting com- age group. The most important diagnosis to plaint in the accident and emergency (A&E) exclude is that of ectopic pregnancy; this department. It can occur in all age groups from currently occurs at a rate of 9.6/1000 the young girl to the elderly woman. Vaginal pregnancies.3 An average district general hospi- bleeding occurs in up to 25% of all pregnancies tal will expect to deal with one ectopic and many of these women present to A&E pregnancy a week, although not all present via rather than to their general practitioner (GP) A&E. This condition still kills women and or antenatal clinic because of 24 hour access.' caused nine maternal deaths in the UK in In one teaching hospital 0.7% of patients seen 1991-93.' Unfortunately clinical diagnosis has in one year presented with bleeding in early poor sensitivity, around 50%.4 There are a wide pregnancy.' The main aim of management in range of clinical presentations ranging from the the A&E department is to identify potentially classic collapsed patient with a haemoperito- life threatening conditions and to separate neum, to vaginal spotting. The safest approach those that require urgent gynaecological refer- is to have a high degree of suspicion in each ral from those that can be managed by the GP patient. -

Bleeding in Early Pregnancy
Bleeding in Early Pregnancy Sara Alhaddab Bayan Alnooh Is it important?? Yes because it can cause maternal death Aims: 1- To know that bleeding in early pregnancy is common and the differential diagnoses are extensive. 2- To critically assess the women with early pregnancy bleeding as this can kill the women. v The underlying reasons of bleeding in early pregnancy: Ectopic pregnancy “most Miscarriage Local causes: in the cervix common” (polyps, infections or cancer), Trauma (RTA) v Pregnancy Loss: - Definition: Termination of the conceptus from the time of conception till the time of fetal viability (24 weeks). Why not 20 weeks? • Biochemical pregnancy • Clinical pregnancy Viability: - Fetal weight >500 grams - Incidence: 15-20% of clinically recognized, - Can be much higher if consider chemical pregnancies, before clinical recognition • Miscarriage is spontaneous while abortion is induced either by the doctor or the mother. • Miscarriage or abortion is loss of pregnancy before 20 weeks which is the period of fetal viability (period of viability: can I resuscitate the fetus or not? Can he survive?) • Because our country is following the WHO so we will say loss of pregnancy before 24 weeks (instead of 20 Ws) is miscarriage/abortion. • Bleeding after 24 weeks is considered “antepartum hemorrhage” • Biochemical pregnancy: by testing B HCG either in urine (urine pregnancy test) or blood with no sign of pregnancy in the US • Clinical pregnancy: signs of pregnancy in US (first sign is the gestational sac). v Pathology: - Hemorrhage into the decidua basalis. - Necrotic changes and inflammation in the tissue, adjacent to the bleeding. - Detachment of the conceptus. -
Conditions and Problems in Pregnancy 75 Avoid Lifting Heavy Objects
Conditions and problems in pregnancy 7 7 Your body goes through a lot of changes during pregnancy. Sometimes these C changes can cause you discomfort or in pregnancy onditions and problems irritation, and you may be worried about what is happening to you. There is usually nothing to worry about, but you should mention anything that concerns you to your midwife or doctor. If you have concerns or questions in between appointments write them down in the “what matters to me” section of your maternity hand-held record (MHHR) and discuss with your midwife or doctor at your next appointment. If you think that something may be seriously wrong, trust your own judgement and get in touch with your midwife or doctor straight away. This chapter describes some of the minor and more serious health problems and gives advice on how to deal with them and when you should get help. Problems in early need a second scan to check How to avoid backache pregnancy that all is well. • Avoid lifting heavy objects. Most women feel well in Common minor • Bend your knees and keep early pregnancy but can problems feel uncomfortable. Some your back straight when women describe a pain low Backache lifting or picking something down in the abdomen similar As your baby grows, the up from the floor. to a period pain. This does hollow in your lower back may not necessarily mean that become more pronounced, something is wrong, but if the and this can also cause pain is more than discomfort backache. During pregnancy, or if there is any bleeding, your your ligaments become softer midwife or GP should refer and stretch to prepare you for you for a scan in the early labour. -
Sporadic Miscarriage: Evidence to Provide Effective Care
Series Miscarriage 2 Sporadic miscarriage: evidence to provide effective care Arri Coomarasamy, Ioannis D Gallos, Argyro Papadopoulou, Rima K Dhillon-Smith, Maya Al-Memar, Jane Brewin, Ole B Christiansen, Mary D Stephenson, Olufemi T Oladapo, Chandrika N Wijeyaratne, Rachel Small, Phillip R Bennett, Lesley Regan, Mariëtte Goddijn, Adam J Devall, Tom Bourne, Jan J Brosens, Siobhan Quenby Lancet 2021; 397: 1668–74 The physical and psychological effect of miscarriage is commonly underappreciated. The journey from diagnosis of Published Online miscarriage, through clinical management, to supportive aftercare can be challenging for women, their partners, and April 26, 2021 caregivers. Diagnostic challenges can lead to delayed or ineffective care and increased anxiety. Inaccurate diagnosis of https://doi.org/10.1016/ a miscarriage can result in the unintended termination of a wanted pregnancy. Uncertainty about the therapeutic S0140-6736(21)00683-8 effects of interventions can lead to suboptimal care, with variations across facilities and countries. For this Series See Editorial page 1597 paper, we have developed recommendations for practice from a literature review, appraisal of guidelines, and expert This is the second in a Series of three papers about miscarriage group discussions. The recommendations are grouped into three categories: (1) diagnosis of miscarriage, Tommy’s National Centre for (2) prevention of miscarriage in women with early pregnancy bleeding, and (3) management of miscarriage. We Miscarriage Research, Institute recommend that every country reports annual aggregate miscarriage data, similarly to the reporting of stillbirth. Early of Metabolism and Systems pregnancy services need to focus on providing an effective ultrasound service, as it is central to the diagnosis of Research, University of miscarriage, and be able to provide expectant management of miscarriage, medical management with mifepristone Birmingham, Birmingham, UK (Prof A Coomarasamy MD, and misoprostol, and surgical management with manual vacuum aspiration. -

Vaginal Bleeding in the Early Stages of Pregnancy
n LEARNING OBJECTIVES: • Tex t here • Tex t here CME • Tex t here • Tex t here CE n COMPLETE THE POSTTEST: Page xx n ADDITIONAL CME/CE: Pages xx FEATURE Turn to page 27 for additional information on this month’s CME/CE courses. KIMBERLY D. WALKER; KATHY DEXTER, MLS, MHA, MPA, PA-C; AND BONNIE A. DADIG, ED.D., PA-C Vaginal bleeding in the early stages of pregnancy A number of potential causes of early pregnancy vaginal bleeding must be considered when deciding what type of management is required. p to 25% of all women in the early stages of pregnancy will experience vaginal bleeding Uor spotting. Even more sobering, half of those women will go on to experience intrauterine fetal demise prior to the 20th week.1 To reduce morbidity and mortality, the astute practitioner should be able to effectively assess and manage a patient with vaginal bleeding in the first half of her pregnancy. Causes Appropriate assessment and management of the patient requires an understanding of the differential diagnoses of vaginal bleeding in early pregnancy (Table 1). Fifty percent of early pregnancy bleeds— defined as bleeding at< 20 weeks’ gestation—occur in viable intrauterine pregnancies (IUP). A total of 30% to 50% of early pregnancy bleeds indicate fetal demise, In an ectopic pregnancy, 7% of which are attributable to ectopic pregnancy the embryo (shown) (EP), and fewer than 1% are caused by trophoblastic implants outside the 2 uterine cavity. disease and/or lesions of the cervix/vagina. While chromosomal abnormalities are the most © SCIENCE SOURCE / BIOPHOTO ASSOCIATES common cause of fetal demise and subsequent 1 THE CLINICAL ADVISOR • APRIL 2013 • www.ClinicalAdvisor.comwww.ClinicalAdvisor.com • THE CLINICAL ADVISOR • APRIL 2013 1 CME CE EARLY PREGNANCY BLEEDING The patient will present with vaginal bleeding and mild-to-moderate subrapubic or midline lower abdominal pain that may radiate to the lower back.