Benthic Invertebrates Monitoring of the Muling River Basin in Northeast China
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Characteristics of Nitrate in Major Rivers and Aquifers of the Sanjiang Plain, China
View Online / Journal Homepage Journal of Dynamic Article LinksC< Environmental Monitoring Cite this: DOI: 10.1039/c2em30032j www.rsc.org/jem PAPER Characteristics of nitrate in major rivers and aquifers of the Sanjiang Plain, China Yingjie Cao,ab Changyuan Tang,*b Xianfang Song,a Changming Liua and Yinghua Zhanga Received 16th January 2012, Accepted 6th July 2012 DOI: 10.1039/c2em30032j À The characteristics of nitrate (NO3 ) in major rivers and aquifers of the Sanjiang Plain, China were investigated by hydrogeochemical conditions, nitrogen isotope technique and CFCs trace. An overall À understanding on the sources and fate of NO3 in the surface water and the groundwater was obtained. À The NO3 concentrations in the surface water were low and no samples exceeds the WTO standards. À However, 11.4% of the groundwater samples exceeded the WTO standards, indicating local NO3 pollution in rural areas. Redox condition analysis revealed that most of the surface water had oxic condition, while for the shallow groundwater (mean well depth smaller than 30 m), the redox condition began to change into anoxic zone, and the deep groundwater (mean well depth larger than 50 m) 15 showed strong anoxic condition. The d N-NO3 data indicated soil N and fertilizer contributed the À major sources in the surface water, and NO3 in the groundwater mainly showed a manure origin. In the Songhua–Heilong River, dilution effect was dominating, while for the Wusuli River, it showed that À À mix with water contained excess of NO3 resulted in the NO3 concentration increased along the river. À Additionally, the NO3 transportation in the groundwater was analyzed by groundwater ages derived À from environmental tracer (CFCs) data. -

Saving the Flagship Species of North-East Asia
North-East Asian Subregional Programme for Environmental Cooperation (NEASPEC) SAVING THE FLAGSHIP SPECIES THE FLAGSHIP SAVING SAVING THE FLAGSHIP SPECIES OF NORTH-EAST ASIA OF NORTH-EAST ASIA United Nations ESCAP United Nations Economic and Social Commission for Asia and the Pacific Environment and Sustainable Development Division United Nations Building Rajadamnern Nok Avenue Nature Conservation Strategy of NEASPEC Bangkok 10200 Thailand Tel: (662) 288-1234; Fax: (662) 288-1025 E-mail: [email protected] E-mail: [email protected] Website: <http://www.unescap.org/esd> United Nations ESCAP ECONOMIC AND SOCIAL COMMISSION FOR ASIA AND THE PACIFIC ESCAP is the regional development arm of the United Nations and serves as the main economic and social development centre for the United Nations in Asia and the Pacific. Its mandate is to foster cooperation between its 53 members and 9 associate members. ESCAP provides the strategic link between global and country-level programmes and issues. It supports the Governments of the region in consolidating regional positions and advocates regional approaches to meeting the region’s unique socio-economic challenges in a globalizing world. The ESCAP office is located in Bangkok, Thailand. Please visit our website at www.unescap.org for further information. Saving the Flagship Species The grey shaded area of the map represents the members and associate members of ESCAP of North-East Asia: United Nations publication Nature Conservation Strategy of NEASPEC Copyright© United Nations 2007 ST/ESCAP/2495 -

The Effects of Forest Area Changes on Extreme Temperature Indexes Between the 1900S and 2010S in Heilongjiang Province, China
remote sensing Article The Effects of Forest Area Changes on Extreme Temperature Indexes between the 1900s and 2010s in Heilongjiang Province, China Lijuan Zhang 1,*, Tao Pan 1, Hongwen Zhang 2, Xiaxiang Li 1 ID and Lanqi Jiang 3,4,5 1 Key Laboratory of Remote Sensing Monitoring of Geographic Environment, Harbin Normal University, Harbin 150025, China; [email protected] (T.P.); [email protected] (X.L.) 2 Key Laboratory of Land Surface Process and Climate Change in Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou 730000, China; [email protected] 3 Innovation and Opening Laboratory of Regional Eco-Meteorology in Northeast, China Meteorological Administration, Harbin 150030, China; [email protected] 4 Meteorological Academician Workstation of Heilongjiang Province, Harbin 150030, China 5 Heilongjiang Province Institute of Meteorological Sciences, Harbin 150030, China * Correspondence: [email protected] Received: 29 October 2017; Accepted: 6 December 2017; Published: 9 December 2017 Abstract: Land use and land cover changes (LUCC) are thought to be amongst the most important impacts exerted by humans on climate. However, relatively little research has been carried out so far on the effects of LUCC on extreme climate change other than on regional temperatures and precipitation. In this paper, we apply a regional weather research and forecasting (WRF) climate model using LUCC data from Heilongjiang Province, that was collected between the 1900s and 2010s, to explore how changes in forest cover influence extreme temperature indexes. Our selection of extreme high, low, and daily temperature indexes for analysis in this study enables the calculation of a five-year numerical integration trail with changing forest space. -

Palaeontology and Biostratigraphy of the Lower Cretaceous Qihulin
Dissertation Submitted to the Combined Faculties for the Natural Sciences and for Mathematics of the Ruperto-Carola University of Heidelberg, Germany for the degree of Doctor of Natural Sciences presented by Master of Science: Gang Li Born in: Heilongjiang, China Oral examination: 30 November 2001 Gedruckt mit Unterstützung des Deutschen Akademischen Austauschdienstes (Printed with the support of German Academic Exchange Service) Palaeontology and biostratigraphy of the Lower Cretaceous Qihulin Formation in eastern Heilongjiang, northeastern China Referees: Prof. Dr. Peter Bengtson Prof. Pei-ji Chen This manuscript is produced only for examination as a doctoral dissertation and is not intended as a permanent scientific record. It is therefore not a publication in the sense of the International Code of Zoological Nomenclature. Abstract The purpose of the study was to provide conclusive evidence for a chronostratigraphical assignment of the Qihulin Formation of the Longzhaogou Group exposed in Mishan and Hulin counties of eastern Heilongjiang, northeastern China. To develop an integrated view of the formation, all collected fossil groups, i.e. the macrofossils (ammonites and bivalves) and microfossils (agglutinated foraminifers and radiolarians) have been studied. The low-diversity ammonite fauna consists of Pseudohaploceras Hyatt, 1900, and Eogaudryceras Spath, 1927, which indicate a Barremian–Aptian age. The bivalve fauna consists of eight genera and 16 species. The occurrence of Thracia rotundata (J. de C: Sowerby) suggests an Aptian age. The agglutinated foraminifers comprise ten genera and 16 species, including common Lower Cretaceous species such as Ammodiscus rotalarius Loeblich & Tappan, 1949, Cribrostomoides? nonioninoides (Reuss, 1836), Haplophragmoides concavus (Chapman, 1892), Trochommina depressa Lozo, 1944. The radiolarians comprise ten genera and 17 species, where Novixitus sp., Xitus cf. -

DRAINAGE BASINS of the SEA of OKHOTSK and SEA of JAPAN Chapter 2
60 DRAINAGE BASINS OF THE SEA OF OKHOTSK AND SEA OF JAPAN Chapter 2 SEA OF OKHOTSK AND SEA OF JAPAN 61 62 AMUR RIVER BASIN 66 LAKE XINGKAI/KHANKA 66 TUMEN RIVER BASIN Chapter 2 62 SEA OF OKHOTSK AND SEA OF JAPAN This chapter deals with major transboundary rivers discharging into the Sea of Okhotsk and the Sea of Japan and their major transboundary tributaries. It also includes lakes located within the basins of these seas. TRANSBOUNDARY WATERS IN THE BASINS OF THE SEA OF OKHOTSK AND THE SEA OF JAPAN1 Basin/sub-basin(s) Total area (km2) Recipient Riparian countries Lakes in the basin Amur 1,855,000 Sea of Okhotsk CN, MN, RU … - Argun 164,000 Amur CN, RU … - Ussuri 193,000 Amur CN, RU Lake Khanka Sujfun 18,300 Sea of Japan CN, RU … Tumen 33,800 Sea of Japan CN, KP, RU … 1 The assessment of water bodies in italics was not included in the present publication. 1 AMUR RIVER BASIN o 55 110o 120o 130o 140o SEA OF Zeya OKHOTSK R U S S I A N Reservoir F E mur D un A E mg Z A e R Ulan Ude Chita y ilka a A a Sh r od T u Ing m n A u I Onon g ya r re A Bu O n e N N Khabarovsk Ulaanbaatar Qiqihar i MONGOLIA a r u u gh s n s o U CHIN A S Lake Khanka N Harbin 45o Sapporo A Suj fu Jilin n Changchun SEA O F P n e JA PA N m Vladivostok A Tu Kilometres Shenyang 0 200 400 600 The boundaries and names shown and the designations used on this map Ch’ongjin J do not imply official endorsement or acceptance by the United Nations. -
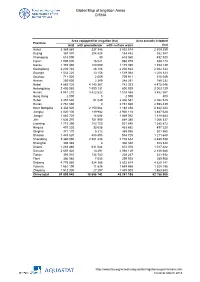
Global Map of Irrigation Areas CHINA
Global Map of Irrigation Areas CHINA Area equipped for irrigation (ha) Area actually irrigated Province total with groundwater with surface water (ha) Anhui 3 369 860 337 346 3 032 514 2 309 259 Beijing 367 870 204 428 163 442 352 387 Chongqing 618 090 30 618 060 432 520 Fujian 1 005 000 16 021 988 979 938 174 Gansu 1 355 480 180 090 1 175 390 1 153 139 Guangdong 2 230 740 28 106 2 202 634 2 042 344 Guangxi 1 532 220 13 156 1 519 064 1 208 323 Guizhou 711 920 2 009 709 911 515 049 Hainan 250 600 2 349 248 251 189 232 Hebei 4 885 720 4 143 367 742 353 4 475 046 Heilongjiang 2 400 060 1 599 131 800 929 2 003 129 Henan 4 941 210 3 422 622 1 518 588 3 862 567 Hong Kong 2 000 0 2 000 800 Hubei 2 457 630 51 049 2 406 581 2 082 525 Hunan 2 761 660 0 2 761 660 2 598 439 Inner Mongolia 3 332 520 2 150 064 1 182 456 2 842 223 Jiangsu 4 020 100 119 982 3 900 118 3 487 628 Jiangxi 1 883 720 14 688 1 869 032 1 818 684 Jilin 1 636 370 751 990 884 380 1 066 337 Liaoning 1 715 390 783 750 931 640 1 385 872 Ningxia 497 220 33 538 463 682 497 220 Qinghai 371 170 5 212 365 958 301 560 Shaanxi 1 443 620 488 895 954 725 1 211 648 Shandong 5 360 090 2 581 448 2 778 642 4 485 538 Shanghai 308 340 0 308 340 308 340 Shanxi 1 283 460 611 084 672 376 1 017 422 Sichuan 2 607 420 13 291 2 594 129 2 140 680 Tianjin 393 010 134 743 258 267 321 932 Tibet 306 980 7 055 299 925 289 908 Xinjiang 4 776 980 924 366 3 852 614 4 629 141 Yunnan 1 561 190 11 635 1 549 555 1 328 186 Zhejiang 1 512 300 27 297 1 485 003 1 463 653 China total 61 899 940 18 658 742 43 241 198 52 -

A Study of the Interrelation Between Surface Water and Groundwater Using Isotopes and Chlorofluorocarbons in Sanjiang Plain, Northeast China
Environ Earth Sci (2014) 72:3901–3913 DOI 10.1007/s12665-014-3279-5 ORIGINAL ARTICLE A study of the interrelation between surface water and groundwater using isotopes and chlorofluorocarbons in Sanjiang plain, Northeast China Bing Zhang • Xianfang Song • Yinghua Zhang • Dongmei Han • Changyuan Tang • Lihu Yang • Zhongliang Wang • Tingyi Liu Received: 14 June 2013 / Accepted: 7 April 2014 / Published online: 27 April 2014 Ó Springer-Verlag Berlin Heidelberg 2014 Abstract Surface water and groundwater are the main recharged from Songhua river. The combination of stable water resources used for drinking and production. Assess- isotopes, tritium, and CFCs was an effectively method to ments of the relationship between surface water and study the groundwater ages and interrelation between sur- groundwater provide information for water resource man- face water and groundwater. Practically, the farmlands near agement in Sanjiang plain, Northeast China. The surface the river and under foot of the mountain could be culti- water (river, lake, and wetland) and groundwater were vated, but the farmlands in the central plain should be sampled and analyzed for stable isotopic (dD, d18O) controlled. composition, tritium, and chlorofluorocarbons concentra- tions. The local meteoric water line is dD = 7.3d18O–6.7. Keywords Hydrogen and oxygen isotopes Á The tritium (T) and chlorofluorocarbon (CFC) contents in Chlorofluorocarbons Á Surface water Á Groundwater Á groundwater were analyzed to determine the groundwater Sanjiang plain ages. Most groundwater were modern water with the ages \50 years. The groundwaters in mountain area and near rivers were younger than in the central plain. The oxygen Introduction isotope (d18O) was used to quantify the relationship between surface water and groundwater. -

Jews in China---- a Presentation by Archie Ossin
Jews in China---- a presentation by Archie Ossin April 2001 –Jewish Genealogy Society – Orlando, FL Oct 2001-Rhoda Goldman Plaza-San Francisco, CA Dec 2001 – 39ers Club – Orlando, FL July 2002– Dartmouth College-Berdakin-Ossinovsky Family Reunion- Hanover, NH April 2003 - Kinneret - Orlando, FL April 2005 Seminole Community College, Lake Mary Florida Feb 2006 Seminole Community College, Lake Mary, FL Aug 2006 – UC Santa Barbara – Berdakin – Ossinovsky-Skidelsky fam Reunion April 17, 2007- Rishonah Chaverot Hadassah – Cong Reform Judaism Orlando © Jan 2001 Archie Ossin 6th-11th Century Jewish Merchants and Traders come back and forth to China on Silk route 11 th Century First Group of Jews (70 families) come to Kaifeng to stay. Kaifeng is Capital of China during Song Dynasty, Population 1.5 Million 1163 First Synagogue built in Kaifeng 1492 Spanish Inquisition 1500 Kaifeng Jewish population peaks at 5000 Kaifeng repeatedly destroyed by flooding of Yellow River Kublai Khan moved capital to Peking Jews always intermarried in Kaifeng and Jewish traditions maintained for 700 years © Jan 2001 Archie Ossin 16 th Century -Decline of Jewish community- intermarriage and assimilation 1605 Jewish community first discovered by Christian Missionaires. Jesuit Priest, Father Matteo Ricci, wrote about the Jewish Community, igniting a continuing interest in this community by missionaries and scholars ever since. 1792 Russia Jewish Pale established in areas annexed in Poland 1800’s Kaifeng Synagogue repaired and rebuilt several times until the 19 th Century when the last Rabbi died and Hebrew no longer taught. © Jan 2001 Archie Ossin 1842 Opium wars open China to Western Society 1842 China First Sephardic Jews arrive in Shanghai 1850 China-- First Jewish office opened by Elias Sassoon son of David Sassoon, an Iraqi Jew, working in Bombay as a Commodities Dealer. -

Harbin Bank Co., Ltd
HARBIN BANK CO., LTD. (A joint stock company incorporated in the People’s Republic of China with limited liability) Stock Code: 6138 2013 Annual Report 2013 Annual Report The Company holds the Finance Permit No. B0306H223010001 approved by the China Banking Regulatory Commission and obtains the Corporate Business License No. 230100100006877 approved by Harbin Administration for Industry and Commerce. The Company is not an authorized institution within the meaning of the Hong Kong Banking Ordinance (Chapter 155 of the Laws of Hong Kong), not subject to the supervision of the Hong Kong Monetary Authority, and not authorized to carry on banking/deposit-taking business in Hong Kong. Contents Defi nitions 2 Company Profi le 3 Harbin Bank Co., Ltd. Annual Report 2013 Summary of Accounting Data and Financial Indicators 8 Chairman’s Statement 12 Management Discussion and Analysis 15 Changes in Share Capital and Information on Shareholders 70 Report of the Board of Directors 75 Report of the Board of Supervisors 82 Important Events 86 Internal Control 89 Corporate Governance Report 91 Directors, Supervisors, Senior Management, Employees and Organizations 113 Financial Statements 128 Documents for Inspection 264 Defi nitions In this report, unless the context otherwise requires, the following terms shall have the meanings set out below. “Company”, “our Bank”, “Bank”, Harbin Bank Co., Ltd. (哈爾濱銀行股份有限公司), a joint stock or “We” company incorporated in the PRC on 25 July 1997 with limited liability in accordance with PRC laws and, unless context indicates -

The Report on the Quantity Monitoring, Threatening Factors and Protection Proposals of Breeding Population of Oriental White Stork in Sanjiang Plain
The Report on the Quantity Monitoring, Threatening Factors and Protection Proposals of Breeding Population of Oriental White Stork in Sanjiang Plain Authors:Wang qiang, Ma zhilong, E mingju Consultant: Liu peiqi Contents Authors:Wang qiang, Ma zhilong, E mingju................................................................................... 1 Consultant: Liu peiqi...........................................................................................................................1 Introduction......................................................................................................................................... 3 1 The historical distribution area and population quantity of Oriental White Stork..........................4 1.1 Breeding ground distribution................................................................................................ 4 1.2 Migration route......................................................................................................................6 1.2.1 Heilongjiang province................................................................................................6 1.2.2 Jilin province..............................................................................................................6 1.2.3 Inner Mongolia province............................................................................................7 2 The comparison of the number of breeding population of Oriental White Storks in nature reserves of Sanjiang Plain.................................................................................................................. -

8. Population of Capital Cities and Cities of 100 000 Or More
8. Population of capital cities and cities of 100 000 or more inhabitants: latest available year, 1990 - 2009 Population des capitales et des villes de 100 000 habitants ou plus : dernière année disponible, 1990 - 2009 City proper - Ville proprement dite Urban agglomeration - Agglomération urbaine Continent, country or area, date, code and city Population Population Surface Surface Continent, pays ou zone, date, code et ville Both sexes Both sexes Male - Female - area - Male - Female - area - - Les deux - Les deux Masculin Féminin Superficie Masculin Féminin Superficie sexes sexes (km²) (km²) AFRICA - AFRIQUE Algeria - Algérie 25 VI 1998 (CDJC) ALGIERS (EL DJAZAIR) ..................................... 1 569 897 ... ... ... ... ... ... ... Annaba ................................................................ 352 523 ... ... ... ... ... ... ... Batna ................................................................... 246 800 ... ... ... ... ... ... ... Béchar ................................................................. 134 523 ... ... ... ... ... ... ... Bejaïa .................................................................. 144 405 ... ... ... ... ... ... ... Beskra ................................................................. 177 060 ... ... ... ... ... ... ... Bordj Bou Arreridj ................................................ 129 004 ... ... ... ... ... ... ... Bordj el Kiffan ...................................................... 103 690 ... ... ... ... ... ... ... Ech Cheliff (El Asnam) ....................................... -

Questionnaire of Community and Forest Farm
IPP779 V1 Public Disclosure Authorized Landscape Approach to Wildlife Conservation in Northeast China Project Public Disclosure Authorized Social Impact Assessment Report Public Disclosure Authorized Public Disclosure Authorized March, 2015 Executive Summary In order to promote the protection and management of the Siberian tiger and its habitat, the State Forestry Administration and the World Bank jointly applied for the GEF “Landscape Approach to Wildlife Conservation in Northeast China Project”, and got approval in principle on Feb, 29th, 2012. This project is implemented in Northeast China, at the junction area of Heilongjiang Province and Jilin Province, close to the Primorsky Region of Russia and Hamgyong Province of North Korea. It involves Hunchun City, Wangqing County, Dongning County, and Muling County, with a total area of 13879.26 square kilometers. The aggregate amount of the project is 18 million US dollars. The undertaking units of the project include Jilin Forestry Department, Heilongjiang Forestry Department, and the General Bureau of Heilongjiang Forest Industry. Contents of the project mainly include conservation station construction, new nature reserve construction or expansion, tiger-friendly forest management activities and supplementary feeding stations construction. The implementation area all belong to the state-owned forest farms affiliated to the General Bureau of Heilongjiang Forest Industry, Heilongjiang Forestry Department and Jilin Forestry Department. Departments in charge of these involved forest farms