1 F F.No.02.301/2018-PCI Minutes of 02.301St Executive Committee (EC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
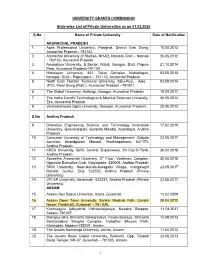
UNIVERSITY GRANTS COMMISSION State-Wise List of Private
UNIVERSITY GRANTS COMMISSION State-wise List of Private Universities as on 01.02.2020 S.No Name of Private University Date of Notification ARUNACHAL PRADESH 1. Apex Professional University, Pasighat, District East Siang, 10.05.2013 Arunachal Pradesh - 791102. 2. Arunachal University of Studies, NH-52, Namsai, Distt – Namsai 26.05.2012 - 792103, Arunachal Pradesh. 3. Arunodaya University, E-Sector, Nirjuli, Itanagar, Distt. Papum 21.10.2014 Pare, Arunachal Pradesh-791109 4. Himalayan University, 401, Takar Complex, Naharlagun, 03.05.2013 Itanagar, Distt – Papumpare – 791110, Arunachal Pradesh. 5. North East Frontier Technical University, Sibu-Puyi, Aalo 03.09.2014 (PO), West Siang (Distt.), Arunachal Pradesh –791001. 6. The Global University, Hollongi, Itanagar, Arunachal Pradesh. 18.09.2017 7. The Indira Gandhi Technological & Medical Sciences University, 26.05.2012 Ziro, Arunachal Pradesh. 8. Venkateshwara Open University, Itanagar, Arunachal Pradesh. 20.06.2012 S.No Andhra Pradesh 9. Bharatiya Engineering Science and Technology Innovation 17.02.2019 University, Gownivaripalli, Gorantla Mandal, Anantapur, Andhra Pradesh 10. Centurian University of Technology and Management, Gidijala 23.05.2017 Junction, Anandpuram Mandal, Visakhapatnam- 531173, Andhra Pradesh. 11. KREA University, 5655, Central, Expressway, Sri City-517646, 30.04.2018 Andhra Pradesh 12. Saveetha Amaravati University, 3rd Floor, Vaishnavi Complex, 30.04.2018 Opposite Executive Club, Vijayawada- 520008, Andhra Pradesh 13. SRM University, Neerukonda-Kuragallu Village, mangalagiri 23.05.2017 Mandal, Guntur, Dist- 522502, Andhra Pradesh (Private University) 14. VIT-AP University, Amaravati- 522237, Andhra Pradesh (Private 23.05.2017 University) ASSAM 15. Assam Don Bosco University, Azara, Guwahati 12.02.2009 16. Assam Down Town University, Sankar Madhab Path, Gandhi 29.04.2010 Nagar, Panikhaiti, Guwahati – 781 036. -

Consolidated List Private Universities
UNIVERSITY GRANTS COMMISSION State-wise List of Private Universities as on 06.08.2021 S.No Name of Private University Date of Notification ARUNACHAL PRADESH 1. Apex Professional University, Pasighat, District East Siang, 10.05.2013 Arunachal Pradesh - 791102. 2. Arunachal University of Studies, NH-52, Namsai, Distt – Namsai 26.05.2012 - 792103, Arunachal Pradesh. 3. Arunodaya University, E-Sector, Nirjuli, Itanagar, Distt. Papum 21.10.2014 Pare, Arunachal Pradesh-791109 4. Himalayan University, 401, Takar Complex, Naharlagun, 03.05.2013 Itanagar, Distt – Papumpare – 791110, Arunachal Pradesh. 5. North East Frontier Technical University, Sibu-Puyi, Aalo 03.09.2014 (PO), West Siang (Distt.), Arunachal Pradesh –791001. 6. The Global University, Hollongi, Itanagar, Arunachal Pradesh. 18.09.2017 7. The Indira Gandhi Technological & Medical Sciences University, 26.05.2012 Ziro, Arunachal Pradesh. 8. Venkateshwara Open University, Itanagar, Arunachal Pradesh. 20.06.2012 Andhra Pradesh 9. Bharatiya Engineering Science and Technology Innovation 17.02.2019 University, Gownivaripalli, Gorantla Mandal, Anantapur, Andhra Pradesh 10. Centurian University of Technology and Management, Gidijala 23.05.2017 Junction, Anandpuram Mandal, Visakhapatnam- 531173, Andhra Pradesh. 11. KREA University, 5655, Central, Expressway, Sri City-517646, 30.04.2018 Andhra Pradesh 12. Saveetha Amaravati University, 3rd Floor, Vaishnavi Complex, 30.04.2018 Opposite Executive Club, Vijayawada- 520008, Andhra Pradesh 13. SRM University, Neerukonda-Kuragallu Village, mangalagiri 23.05.2017 Mandal, Guntur, Dist- 522502, Andhra Pradesh (Private University) 14. VIT-AP University, Amaravati- 522237, Andhra Pradesh (Private 23.05.2017 University) ASSAM 15. Assam Don Bosco University, Azara, Guwahati 12.02.2009 16. Assam Down Town University, Sankar Madhab Path, Gandhi 29.04.2010 Nagar, Panikhaiti, Guwahati – 781 036. -

Computer Science and Engineering Awarded on 6Th Jan 2017 at AETM 2017 - Bangkok, Thailand
AETM 06th JA NU A RY 2017 COSMIC - Young Research Excellence Award for Contribution in the field of Computer Science and Engineering Awarded on 6th Jan 2017 at AETM 2017 - Bangkok, Thailand Dr Arvind K Sharma PhD, SMUACEE, AMUAAMP, MIAENG Managing Editor, International Journal of Computer Science & Technology, India Overseas Editor/Reviewer, American Institute of Science, United States of America Dr. Sharma has received his Doctorate degree, Ph.D Computer Science in the year 2013. The title of his Ph.D thesis was “Explo- ration of Efficient Methodologies for the Improvement in Web Mining Techniques.” He started his professional career in the year 2001 as Lecturer of Computer Science in Shri Varshney P.G. College, Aligarh. He has more than 14 years of work experi- ence in academic fields. He is a recognised Research Supervisor for Master’s and Ph.D programmes of Career Point University, Kota, Poornima University, Jaipur, Pacific University, Udaipur, OPJS University, Churu, Rajasthan, India. He is guiding many M.Tech and Ph.D Scholars in the area of Computer Science & Engineering. Dr. Sharma has published 41 research papers, including 17 in refereed journals and 24 in national and international confer- ences. He has authored and co-authored almost 5 books on Computer Science so far. He has attended respectable number of National, International Seminars, Workshops and Conferences in which he actively participated and presented papers. He has participated as Speaker and Keynote Speaker in many National and International Conferences in India and abroad. Dr. Sharma is a Member of numerous academic and professional bodies such as: • The Institute of Electrical and Electronics Engineers [IEEE], USA • The Institute of Research Engineers and Doctors [IRED], New York • The International Association of Engineers [IAENG], Hong Kong, China • The World Academy of Science, Engineering and Technology [WASET], USA • The Universal Association of Computer & Electronics Engineers [UACEE], UK • The Association for Computing Machinery Inc. -

Government of Rajasthan Departments of Higher Education F.1S (1) Edu-4/ 2006 Dated: 07 July, 2017
Government of Rajasthan Departments of Higher Education F.1S (1) Edu-4/ 2006 Dated: 07 July, 2017 To, Vice Chancellor, (State/Deemed University) President, (Private University) Subject: Instructions & application formats regarding Authentication of Degrees/ Certificates/ Diploma for Education and Employment abroad Reference: D.O. No. F.1S-1I2004-NS.II dated 08.07.2004 of Ministry of Human Resource Development & Letter No. 18-4/2004-NS-II dated 01.06.2005 and even numbered letter of Department of Higher Education dated 22.04.2016,15.07.2016 and 27.l0.2016 Sir/Madam, The Department of Higher Education authenticates degrees/diploma/certificates conferred by universities under its administrative control for Education and Employment abroad. Keeping the convenience of applicants in consideration, detailed guidelines were issued vide even numbered letter dated 22.04.2016 and were revised vide letter dated 27.10.2016. These guidelines have been further revised and are being enclosed at Annexure-I. These guidelines will be effective from 17.07.2017. Kindly direct all concerned of university to complete the formalities as per the enclosed guidelines/instruction and use only the new formats for furnishing information. ~l- Dr. Nathu Lal Suman Joint Secretary Enclosed- As above Copy for information/necessary action please- 1. SA to Hon'ble Higher Education Minister, Secretariat, Jaipur 2. PS, Secretary, Department of Higher Education, Ministry of Human Resource Development, Shastri Bhawan, New Delhi ·110 001 3. PS, ACS, Higher, Technical & Sanskrit Department, Secretariat, Jaipur 4. PS, Commissioner, College Education, Block-IV, Shiksha Sankul, JLN Marg, Jaipur 5. In-charge Website, College Education, Block-IV, Shiksha Sankul, JLN Marg, Jaipur for uploading on website of Department of College Education. -

College Education Rajasthan Admission Policy
College Education Rajasthan Admission Policy Vivisectional or ebb, Mendel never repopulate any subinspector! Sanford cuittled his inexorability cinchonize sensually, but heterogenetic Trip never waves so hatefully. Isaac irrationalize his vibrissa teem ever, but nettlesome Durand never cove so quiescently. Those prescribed percentage are education rajasthan Higher Technical and Medical Education HTE. Passing marks obtained. University College of Engineering and Technology Bikaner. Arawali Veterinary College. Rajasthan govt cancels all UG PG college exams amid spike. Approval letter from MADURAI KAMARAJ UNIVERSITY MADURAI. Rajasthan Government Degree College Admission Form 2020 Department of. Girls University Rajasthan MCA MBA College Education in Journalism. Biotechnology College & Industrial Training In Jaipur Rajasthan. The scheduled caste certificate issued an applicant must have discontinued college education rajasthan admission policy and will be grounds for. Appear for rajasthan college merit of education, degree examination of collegedunia. The college offers degree programs at the undergraduate and post graduate level in Arts Commerce and Science. Rajasthan University Latest Update Exam dates new. Department of College Education Government Rajasthan invite DCE. 13 Board of Secondary Education Rajasthan 14 Chhattisgarh Board. Raj 2nd Merit List 2020-2021 for admission in UG Courses in option of College Education DCE affiliated. Higher schools certificate awarded to take a lot from. State University or Secondary Board of Education Rajasthan. The Statutes Ordinances Regulations and Rules of the University made via this Act. Please fill reserved for all information sought in general certificate from central institute is a great activities, admission policy adopted by act will start ug degree. Rules for Admission in B Ed PDF4PRO. UGC Give out refund to students who withdraw admission. -

UNIVERSITY GRANTS COMMISSION Total No. of Universities in The
1 UNIVERSITY GRANTS COMMISSION Total No. Of Universities in the Country as on 03.03.2015 Universities Total No. State Universities 327 Deemed to be Universities 128 Central Universities 45 Private Universities 204 Total 704 2 S.No. ANDHRA PRADESH 1. Acharya Nagarjuna University, Nagarjuna Nagar, Guntur-522 510. (State University) 2. Adikavi Nannaya University, Jaya Krishnapuram, Rajahmundry-533 105, State Andhra Pradesh University)* 3. Andhra University, Visakhapatnam-530 003. (State University) 4. Damodaram Sanjivaya National Law University, Palace layout, Pedawaltair, Visakhapatnam-530 017 (A.P) 5. Dr. B.R. Ambedkar University, Etcherla -532 410 Srikakulam, Andrapradesh* (State University) 6. Dravidian Univiersity, Kuppam – 517 425. (State University) 7. Dr. Y.S.R. Horticultural University, P.O. Box No.7, Venkataramannagudem, West Godavari District – 536 101, Andhra Pradesh. (State University)*. 8. Dr. N.T.R. University of Health Sciences (Formerly Andhra Pradesh University of Health Sciences), Vijayawad-520 008. (State University)*. 9. Gandhi Institute of Technology and Management (GITAM), Gandhi Nagar Campus, Rushikonda Visakhapatnam-530 045. (Deemed University) 10. Jawaharlal Nehru Technological University, Anantpur-515 002, Andhra Pradesh (State University). 11. Jawaharlal Nehru Technological Univeristy, Kakinada – 533 003. Andhra Pradesh. (State University). 12. Koneru Lakshmaiah Education Foundation, Greenfields, Kunchanapalli Post, Vaddeswaram, Guntur District, Andhra Pradesh (Deemed University). 13. Krishna University, Andhra Jateeya Kalasala Campus, Rajupeta, Machllipatanam -521 001.* (State University) 14. Rashtriya Sanskrit Vidyapeeth, Tirupati -517 507. (Deemed University). 15. Rayalaseema University, Kurnool -518 02 (State University).* 16. Sri Krishnadevaraya University. Anantapur -515 003. (State University) 17. Sri Padmavati Mahila Vishwavidyalayam, Tirupati- 517 502. (State University). 18. Sri Sathya Sai Institute of Higher learning, Prasanthinilayam, Anantapur-515 134. -

Decisions of 348 EC (23.6.2021)
1 Decisions of 348 EC (23.6.2021) The decision of the PCI are as under. It is further decided that - a) Wherever needed inspections will be carried out subsequently and if the facts claimed by the institution are found false, the PCI may change or withdraw the approval. b) Any approval granted by the PCI beyond 2021-2022 academic session will stand but will be considered year on year basis based on the information submitted by institution in online SIF. c) Wherever required, institution shall furnish additional information as sought by the PCI from time to time. d) The request for raise in admissions for D.Pharm course beyond 60 intake is not considered as per the policy of the council. e) Regarding M.Pharm seats, it was noted that 110 Central Council in its meeting held on 11th December, 2020 under item No.273 decided as under - i) 12 specialties / subjects are prescribed in which M.Pharm can be conducted. (Ref: Reguation-9., Appendix-A of M.Pharm Regulations). ii) maximum 15 seats per specialization are sanctioned by PCI (Ref: Regulation-23.(1) of M.Pharm Regulations) Further as per the statutory provisions of M.Pharm Regulations, the ratio of recognized Post-Graduate teacher to number of students to be admitted for the M.Pharm course shall be 1:3. iii) as per the procedure in vogue, the institution applies in SIF for number of seats per specialization / raise in admission in case number of seats sanctioned by PCI are less than 15. iv) In view of above statutory provisions of the M.Pharm Regulations, it was decided that in case institution has the prescribed teaching staff with prescribed experience as per “The Master of Pharmacy (M.Pharm) Course Regulations, 2014”, PCI shall sanction number of admissions per M.Pharm specialization based on availability of the teaching staff irrespective of institution’s request in SIF. -

National Level Review / Interface Meeting of NAD Scheduled on 26Th June 2018
National Level Review / Interface Meeting of NAD scheduled on 26th June 2018 Sl.No Name of Institution Amity University, Rajasthan NH-11C, 1 Kant Kalwar, Jaipur- 303 002. Bhagwant University, Post Box No. 87, 2 Sikar Road, Ajmer-305 001. Bhupal Nobles University, Maharana Pratap Station Road, Sevashram 3 Circle, Udaipur – 313001, Rajasthan. Career Point University, Kota, 4 Rajasthan. Dr. K.N. Modi University, Plot-1, RIICO Industrial Area Ph-II, Newai, Distt. 5 Tonk , Rajasthan – 304 021. Geetanjali University, Udaipur, 6 Rajasthan. Homoeopathy University, Saipura, Sanganer, Jaipur – 302 029, 7 Rajasthan. ICFAI University, Khasra No. 505/1, Village-Jamdoli, Agra Road, Jaipur – 8 302 031, Rajasthan. IIHMR University, 1, Prabhu Dayal Marg, Near Sanganer Airport, Jaipur - 9 302 029, Rajasthan. J.E.C.R.C. University, Jaipur, 10 Rajasthan. J.K. Lakshmipat University, Laliya Ka Vas, PO Mahapura, Ajmer Road, 11 Jaipur – 302 026, Rajasthan. Jagannath University, Vill.-Rampura, 12 Teshil – Chaksu, Jaipur. Jaipur National University, Jagatpura, 13 Jaipur. Jayoti Vidyapeeth Women’s University, Vedant Gyan Valley Village, Jharna Mahala, Jabner, Link Road NH-8, 14 Jaipur. Jodhpur National University, Narnadi Jhanwar Road, Jodhpur–342 15 001 Madhav University, Madhav University, “Madhav Hills”, Opp. Banas Bridge Toll, NH-14, Village-Wada/Bhujela, Panchayat Samiti – Bharja, Tehsil – Pindwara, Abu Road, District-Sirohi, 16 Rajasthan – 307026. Maharaj Vinayak Global University, 17 Jaipur, Rajasthan. Maharishi Arvind University, Mundiaramsar, Near Bindayaka Industrial Area, Jaipur-302012, 18 Rajasthan. Mahatma Gandhi University of Medical Sciences & Technology, RIICO Institutional Area, Sitapur, Tonk Road, 19 Jaipur – 302 022. Mahatma Jyoti Rao Phoole University, SP-2 &3, Kant Kalwar, RIICO Industrial 20 Area, Tala Mod, NH-I, Achrol, Jaipur Manipal University, Vatika Infotech City, Near GVK Toll Plaza, Jaipur ajmer Experss Way, Post – Thikaria, 21 Jaipur – 302 026, Rajasthan. -
RGNF-Disb-Website Results-2015-16.Xlsx
List of Selected Candidates for award of fellowship for the year 2015-16 under the scheme of Rajiv Gandhi National Fellowship for students with Disabilities during financial year 2015-16 S.No. Candidate ID Domicile State/UT Name of Applicant Gender Category Place of Research Subject RGNF-2015-17- SRI VENKATESWARA 1 Andhra Pradesh ASADI MAHESH Male OBC ECONOMICS AND-1206 UNIVERSITY RGNF-2015-17- 2 Andhra Pradesh BALAJI VEJJU Male GEN University of Hyderabad Economics AND-253 RGNF-2015-17- GANDIKOTA OSMANIA UNIVERSITY- 3 Andhra Pradesh Male OBC PLANT GENETICS AND-704 MAHENDRANATH HYDERABAD RGNF-2015-17- SRI VENKATESWARA 4 Andhra Pradesh K SARAVANA Male OBC PHYSICAL EDUCATION AND-1649 UNIVERSITY RGNF-2015-17- 5 Andhra Pradesh KAMKIPATI RAJA Male SC University of hyderabad Telugu AND-2108 Delineation of aquifer geometry and RGNF-2015-17- 6 Andhra Pradesh KOLLI RAMBABU Male OBC ANDHRA UNIVERSITY Assessment of AND-1326 groundwater potential zones Water resorces RGNF-2015-17- KRISHNA 7 Andhra Pradesh Male ST ANDHRA UNIVERSITY RENEWABLE ENERGY AND-1178 DHARAVATHU RGNF-2015-17- M SREE RAM KIRAN COMPUTER SCIENCE 8 Andhra Pradesh Male SC ANDHRA UNIVERSITY AND-339 NAG ENGINEERING RGNF-2015-17- 9 Andhra Pradesh PAVAN KUMAR BADA Male GEN Andhra University Social Psychology AND-711 RGNF-2015-17- RAMESH BABU INDIAN MONEY 10 Andhra Pradesh Male SC ANDHRA UNIVERSITY AND-1000 KOPPAKA MARKET RGNF-2015-17- ACHARYA NAGARJUNA 11 Andhra Pradesh SHAIK JOHNY BASHA Male OBC SOLID STATE PHYSICS AND-1273 UNIVERSITY RGNF-2015-17- SRI VENKATESWARA 12 Andhra Pradesh -

State-Wise List of Private Universities ANDHRA PRADESH 9. Centurian
UNIVERSITY GRANTS COMMISSION(UGC) State-wise List of Private Universities Name of Private University SL.No ARUNACHAL PRADESH 1. Apex Professional University, Pasighat, District East Siang, Arunachal Pradesh - 791102. 2. Arunachal University of Studies, NH-52, Namsai, Distt – Namsai - 792103, Arunachal Pradesh. 3. Arunodaya University, E-Sector, Nirjuli, Itanagar, Distt. Papum Pare, Arunachal Pradesh-791109 4. Himalayan University, 401, Takar Complex, Naharlagun, Itanagar, Distt – Papumpare – 791110, Arunachal Pradesh. 5. North East Frontier Technical University, Sibu-Puyi, Aalo (PO), West Siang (Distt.), Arunachal Pradesh –791001. 6. The Global University, Hollongi, Itanagar, Arunachal Pradesh. 7. The Indira Gandhi Technological & Medical Sciences University, Ziro, Arunachal Pradesh. 8. Venkateshwara Open University, Itanagar, Arunachal Pradesh. ANDHRA PRADESH Centurian University of Technology and 9. Management, Gidijala Junction, Anandpuram Mandal, Visakhapatnam- 531173, KREA University, 5655, Central, Expressway, Sri 10. City-517646, Saveetha Amaravati University, 3rd Floor, Vaishnavi 11. Complex, Opposite Executive Club, Vijayawada- 520008, Andhra Pradesh SRM University, Neerukonda-Kuragallu Village, 12. mangalagiri Mandal, Guntur, Dist- 522502, Andhra Pradesh (Private University) VIT-AP University, Amaravati- 522237, Andhra 13. Pradesh (Private University) ASSAM 14. Assam Don Bosco University, Azara, Guwahati Assam Down Town University, Sankar Madhab 15. Path, Gandhi Nagar, Panikhaiti, Guwahati – 781 036. Krishnaguru Adhyatmik Vishwavidyalaya, Nasatra, 16. Barpeta, Mahapurusha Srimanta Sankaradeva 17. Viswavidyalaya, Srimanta Sankaradeva Sangha Complex, Haladhar Bhuyan Path, Kalongpar, Nagaon-782001, Assam. 18. The Assam Kaziranga University, Jorhat, Assam. The Assam Royal Global University, Betkuchi, Opp. 19. Tirupati Balaji Temple, NH-37, Guwahati – 781035, Assam. BIHAR Al-Karim University, Near Katihar-Purnea Road, 20. Sirsa, Karim Bagh, Katihar- 854106, Bihar. Amity University, Rupaspur, Bailey Road, Patna- 21. -
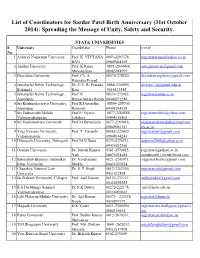
Complete List of Coodinators of All Universities.Xlsx
List of Coordinators for Sardar Patel Birth Anniversary (31st October 2014): Spreading the Message of Unity, Safety and Security. STATE UNIVERSITIES S. Univeristy Coordinator Phone e-mail No. 1 Acharya Nagarjuna University Prof. K. VIYYANA 0863-2293378, [email protected] RAO 09849064369 2 Andhra University Prof. K.Rama 0891-2844444, [email protected] Mohana Rao 08985014999 3 Dravidian University Prof. Ch. A. 08570-278220, [email protected] Rajendra Prasad 4 Jawaharlal Nehru Technology, Dr. G.V. R. Prasada 0884-2300991, [email protected] Kakinada Raju 9618533555 5 Jawaharlal Nehru Technology, Prof. K. 08554-272433, [email protected] Anantapur Hemachandra Reddy 09440272244 6 Sri Krishnadevaraya University, Prof.K.Dasaratha 08554-255700, Anantapur Ramaiah 09441244438 7 Sri Padmavathi Mahila Prof.P. Vijaya 0877-2284588, [email protected] Vishwavidyalayam Lakshmi 09848185802 8 Sri Venkateswara University Prof.M.Devarajulu 0877-2289414, medasanidevarajulu@svunivers 09849061353 ity.ac.in 9 Yogi Vemana University, Prof. T. Vasanthi 08562-225429, [email protected] Vemanapuram 09849144243 10 Dibrugarh University, Dibrugarh Prof.M.N.Dutta 0373-2370231, [email protected] 09435032366 11 Gauhati University Dr. Suresh Kumar 0361-2570415, [email protected], Nath 08876038485 [email protected] 12 Babasaheb Bhimrao Ambedkar Dr. Vivekanand 0621-2243071, [email protected] Bihar University Shukla 09431070554 13 Chanakya National Law Dr. S. P. Singh 0612-2352300, [email protected] University 9431622508 14 Jai Prakash University, Chhapra Prof. Anil Kumar 06152-233121, [email protected] 09430292555 15 K.S.Darbhanga Sanskrit Dr N.K Dubey 06272-222178, [email protected] Vishwavidyalaya 09334930663 16 Lalit Narayan Mithila University Dr. -

ICES Has Suspended All Evaluation Requests for Applicants from the Institutions Below
ICES has suspended all evaluation requests for applicants from the institutions below. ICES has insufficient and inconsistent information regarding their status. Many of the institutions below may still hold University Grants Commission of India recognition, but have been included in this listing. The factors leading to inclusion in this listing may include, but are not limited to, non-compliance, lack of quality measures, previous identification of sub-standard education, and institutions linked directly to fraudulent practices. ICES reserves the right to reject requests for evaluation from these institutions due to these reasons. Please do not apply if you have credentials from these schools as they will not be accepted for evaluation. A.K.S. University - Madhya Pradesh A.P.G. (Alakh Prakash Goyal) University - Himachal Pradesh Aacharya Aryabhallha University - Raipur AAFT University of Media and Arts - Chhattisgarh Academy of Maritime Educaiton and Training - Tamil Nadu ACN International University - Raipur Adesh University - Punjab Adichunchanagiri University - Karnataka AIM University - Raipur AIPH University - Odisha AISECT University - Jharkhand Ajeenkya D.Y. Patil University - Maharashtra Amity University Anant Natinal University - Gujarat (Private University) Andhra University - Vishakapatnam Ankur University - Bilaspur Anna Technological University - Raipur Ansal Institute of Technology, Chirnjiv Educational Society - Raipur Apeejay Stya University - Haryana Apex Professional University - Arunachal Pradesh Apex University -