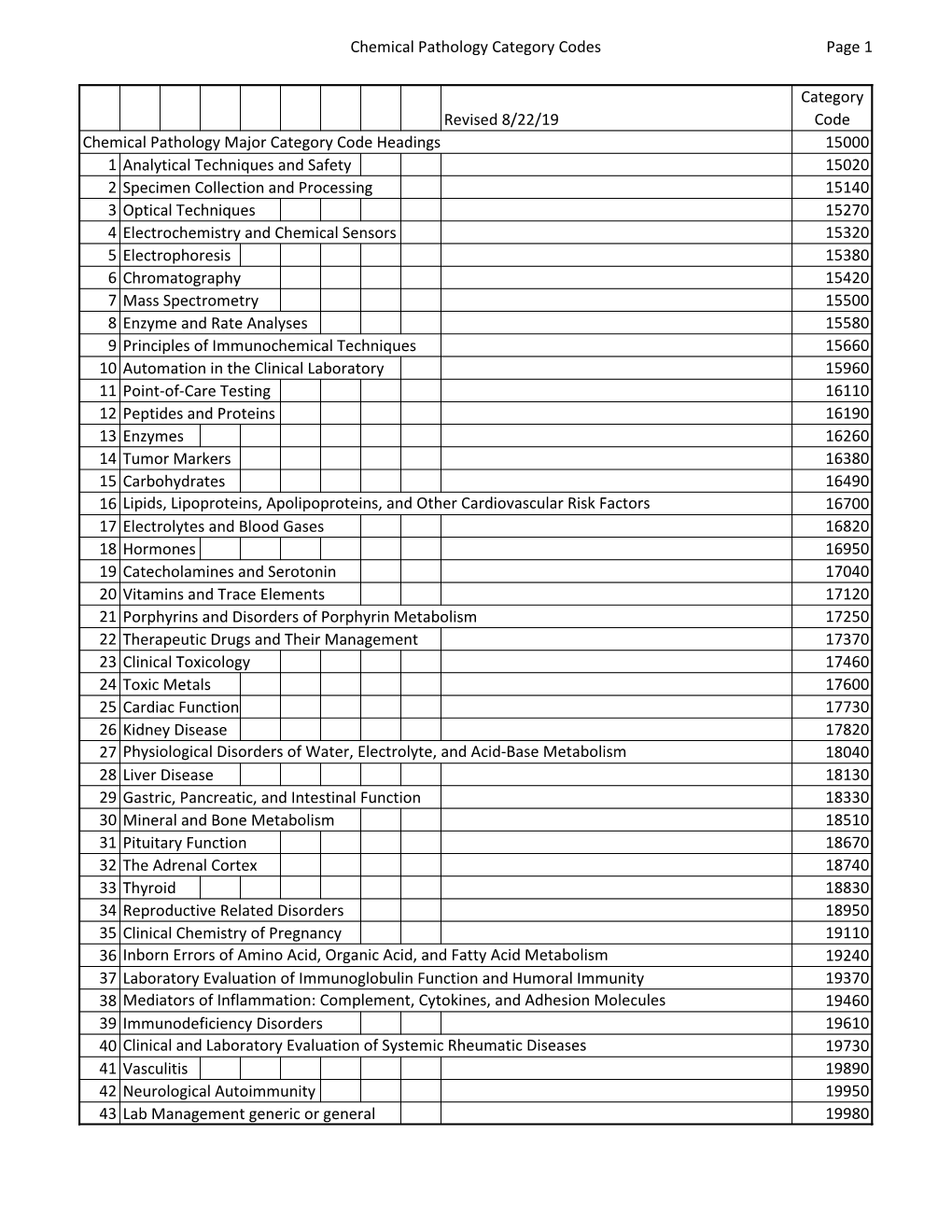
Chemical Pathology Category Codes Page 1 Revised 8/22/19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Severe Metabolic Acidosis in a Patient with an Extreme Hyperglycaemic Hyperosmolar State: How to Manage? Marloes B
Clinical Case Reports and Reviews Case Study ISSN: 2059-0393 Severe metabolic acidosis in a patient with an extreme hyperglycaemic hyperosmolar state: how to manage? Marloes B. Haak, Susanne van Santen and Johannes G. van der Hoeven* Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, the Netherlands Abstract Hyperglycaemic hyperosmolar state (HHS) and diabetic ketoacidosis (DKA) are often accompanied by severe metabolic and electrolyte disorders. Analysis and treatment of these disorders can be challenging for clinicians. In this paper, we aimed to discuss the most important steps and pitfalls in analyzing and treating a case with extreme metabolic disarrangements as a consequence of an HHS. Electrolyte disturbances due to fluid shifts and water deficits may result in potentially dangerous hypernatriema and hyperosmolality. In addition, acid-base disorders often co-occur and several approaches have been advocated to assess the acid-base disorder by integration of the principles of mass balance and electroneutrality. Based on the case vignette, four explanatory methods are discussed: the traditional bicarbonate-centered method of Henderson-Hasselbalch, the strong ion model of Stewart, and its modifications ‘Stewart at the bedside’ by Magder and the simplified Fencl-Stewart approach. The four methods were compared and tested for their bedside usefulness. All approaches gave good insight in the metabolic disarrangements of the presented case. However, we found the traditional method of Henderson-Hasselbalch and ‘Stewart at the bedside’ by Magder most explanatory and practical to guide treatment of the electrolyte disturbances and in exploring the acid-base disorder of the presented case. Introduction This is accompanied by changes in pCO2 and bicarbonate (HCO₃ ) levels, depending on the cause of the acid-base disorder. -

Toxicology and Environmental Teaching Tue 1St Oct 2019
TOXICOLOGY AND ENVIRONMENTAL TEACHING TUE 1ST OCT 2019 CASE ONE The ambulance bring in a 50 year old woman who has been found confused at her home by friends. There is no history available, but she was last seen well around dinner yesterday. She has no known past history and her medications are unknown. Initial Obs: - GCS M 5, E4, V4 pupils large and reactive - SBP 110 - HR 120 - Sats 99% o/a - Afebrile - BSL 8 1. She is confused and denies any ingestion or overdose. She dose state she only took some sleeping medication. What investigations would you do and how would you manage her presentation? Look up NHI: prior hx, community dispensing etc Investigations: Bloods, VBG, ECG Consider differential diagnoses : sepsis, trauma, metabolic 2. You note a community dispensing for 60 x 20mg amitriptyline tablets a few days ago. Her ECG and VBG are shown, please describe and discuss the findings: Potential toxic dose > 10mg/kg onset, toxicity usually rapid onset in 1- 2hrs VBG pH 7.35 PCO2 4.0 HCO3 15 Na 143 K 4.2 Lactate 3.5 - Sinus tachycardia with first-degree AV block (P waves hidden in the T waves, best seen in V1-2). - Broad QRS complexes. - Positive R’ wave in aVR. ECG Features of Sodium-Channel Blockade Interventricular conduction delay — QRS > 100 ms in lead II Right axis deviation of the terminal QRS: o Terminal R wave > 3 mm in aVR o R/S ratio > 0.7 in aVR Patients with tricyclic overdose will also usually demonstrate sinus tachycardia secondary to muscarinic (M1) receptor blockade. -

Reporting Terminology and Codes Chemical Pathology (V3.0)
Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Chemical Pathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards and Guidelines (APUTS) ISBN: Pending State Health Publication Number (SHPN): Pending Online copyright © RCPA 2017 This work (Standards and Guidelines) is copyright. You may download, display, print and reproduce the Standards and Guidelines for your personal, non- commercial use or use within your organisation subject to the following terms and conditions: 1. The Standards and Guidelines may not be copied, reproduced, communicated or displayed, in whole or in part, for profit or commercial gain. 2. Any copy, reproduction or communication must include this RCPA copyright notice in full. 3. No changes may be made to the wording of the Standards and Guidelines including commentary, tables or diagrams. Excerpts from the Standards and Guidelines may be used. References and acknowledgments must be maintained in any reproduction or copy in full or part of the Standards and Guidelines. Apart from any use as permitted under the Copyright Act 1968 or as set out above, all other rights are reserved. Requests and inquiries concerning reproduction and rights should be addressed to RCPA, 207 Albion St, Surry Hills, NSW 2010, Australia. This material contains content from LOINC® (http://loinc.org). The LOINC table, LOINC codes, LOINC panels and forms file, LOINC linguistic variants file, LOINC/RSNA Radiology Playbook, and LOINC/IEEE Medical Device Code Mapping Table are copyright © 1995-2016, Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC) Committee and is available at no cost under the license at http://loinc.org/terms-of-use.” This material includes SNOMED Clinical Terms® (SNOMED CT®) which is used by permission of the International Health Terminology Standards Development Organisation (IHTSDO®). -

IFCC Curriculum
The IFCC Curriculum AUTHORS: R. Greaves, J. M. Smith SECTION AUTHORS: R. Greaves, C Florkowski, L. Langman, J. Sheldon The IFCC Curriculum was developed as part of the IFCC eAcademy project by the Committee for Distance Learning. Authors: Janet M Smith [email protected] Ronda Greaves School of Health and Biomedical Sciences - RMIT University, PO Box 71, Bundoora, Victoria 3083 Australia [email protected] Section authors: Evidence Based Laboratory Medicine Section Chris Florkowski Clinical Biochemistry Unit, Canterbury Health Laboratories, PO Box 151, Christchurch, New Zealand [email protected] Immunology Section Jo Sheldon Protein Reference Unit, St. George's Hospital, Blackshaw Road, London SW17 0NH - UK [email protected] Mass Spectrometry Section Ronda Greaves School of Health and Biomedical Sciences - RMIT University, PO Box 71, Bundoora, Victoria 3083 Australia [email protected] Loralie Langman Department of Laboratory Medicine and Pathology Mayo Clinic 200 2nd Street SW Rochester, MN 55905 USA [email protected] Contacts: [email protected] _________________________________________ The IFCC Curriculum - Rev 0 - December 2017 (effective until further revision) 1 Contents INTRODUCTION .................................................................................................................................. 4 SECTION A: Laboratory Organisation and Management .......................................................... 9 Section A1: Laboratory Principles and Procedures: Learning Objectives ...................... -

BMC Emergency Medicine Biomed Central
BMC Emergency Medicine BioMed Central Research article Open Access An evaluation of the osmole gap as a screening test for toxic alcohol poisoning Larry D Lynd*†1,2, Kathryn J Richardson†2, Roy A Purssell†3,4,5, Riyad B Abu- Laban†3,6, Jeffery R Brubacher†3,4,5, Katherine J Lepik†4 and Marco LA Sivilotti†7 Address: 1Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC, Canada, 2Centre for Health Evaluation and Outcomes Sciences, Providence Health Care, Vancouver, Canada, 3Department of Emergency Medicine, Vancouver General Hospital, Vancouver, Canada, 4British Columbia Drug and Poison Information Centre, Provincial Health Services Authority of BC, Vancouver, Canada, 5Division of Emergency Medicine, Dept. of Surgery, Faculty of Medicine, University of British Columbia; Vancouver; Canada, 6Centre for Clinical Epidemiology and Evaluation, Vancouver Coastal Health Research Institute, Vancouver, Canada and 7Departments of Emergency Medicine and of Pharmacology & Toxicology, Queen's University, Kingston, Canada; and Ontario Poison Centre, Toronto, Canada Email: Larry D Lynd* - [email protected]; Kathryn J Richardson - [email protected]; Roy A Purssell - [email protected]; Riyad B Abu-Laban - [email protected]; Jeffery R Brubacher - [email protected]; Katherine J Lepik - [email protected]; Marco LA Sivilotti - [email protected] * Corresponding author †Equal contributors Published: 28 April 2008 Received: 12 April 2007 Accepted: 28 April 2008 BMC Emergency Medicine 2008, 8:5 doi:10.1186/1471-227X-8-5 This article is available from: http://www.biomedcentral.com/1471-227X/8/5 © 2008 Lynd et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Toxic Alcohol Ingestions: Clinical Features, Diagnosis, and Management
CJASN ePress. Published on November 28, 2007 as doi: 10.2215/CJN.03220807 In-Depth Review Toxic Alcohol Ingestions: Clinical Features, Diagnosis, and Management Jeffrey A. Kraut*† and Ira Kurtz†‡ *Medical and Research Services, UCLA Membrane Biology Laboratory, VHAGLA Healthcare System, and Division of Nephrology VHAGLA Healthcare System, †David Geffen School of Medicine, and ‡Division of Nephrology, UCLA Center for Health Sciences, Los Angeles, California Alcohol-related intoxications, including methanol, ethylene glycol, diethylene glycol, and propylene glycol, and alcoholic ketoacidosis can present with a high anion gap metabolic acidosis and increased serum osmolal gap, whereas isopropanol intoxication presents with hyperosmolality alone. The effects of these substances, except for isopropanol and possibly alcoholic ketoacidosis, are due to their metabolites, which can cause metabolic acidosis and cellular dysfunction. Accumula- tion of the alcohols in the blood can cause an increment in the osmolality, and accumulation of their metabolites can cause an increase in the anion gap and a decrease in serum bicarbonate concentration. The presence of both laboratory abnormalities concurrently is an important diagnostic clue, although either can be absent, depending on the time after exposure when blood is sampled. In addition to metabolic acidosis, acute renal failure and neurologic disease can occur in some of the intoxications. Dialysis to remove the unmetabolized alcohol and possibly the organic acid anion can be helpful in treatment of several of the alcohol-related intoxications. Administration of fomepizole or ethanol to inhibit alcohol dehydrogenase, a critical enzyme in metabolism of the alcohols, is beneficial in treatment of ethylene glycol and methanol intoxication and possibly diethylene glycol and propylene glycol intoxication. -

Emergency Department Alcohol Withdrawal Order
Form Title Emergency Department Alcohol Withdrawal Adult Order Set Form Number 20745Bond © 2018, Alberta Health Services, CKCM This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The license does not apply to content for which the Alberta Health Services is not the copyright owner. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/ Disclaimer: This material is intended for use by clinicians only and is provided on an DVLVZKHUHLVEDVLV$OWKRXJKUHDVRQDEOHHIIRUWVZHUHPDGHWRFRQ¿UPWKHDFFXUDF\RIWKH information, Alberta Health Services does not make any representation or warranty, express, LPSOLHGRUVWDWXWRU\DVWRWKHDFFXUDF\UHOLDELOLW\FRPSOHWHQHVVDSSOLFDELOLW\RU¿WQHVVIRU a particular purpose of such information. This material is not a substitute for the advice of a TXDOL¿HGKHDOWKSURIHVVLRQDO$OEHUWD+HDOWK6HUYLFHVH[SUHVVO\GLVFODLPVDOOOLDELOLW\IRUWKHXVH of these materials, and for any claims, actions, demands or suits arising from such use. Last Name (Legal) First Name (Legal) Preferred Name Last First DOB(dd-Mon-yyyy) Emergency Department Alcohol Withdrawal PHN ULI Same as PHN MRN Adult Order Set Select orders by replacing a () in the associated box Administrative Gender Male Female Non-binary/Prefer not to disclose (X) Unknown Goals of Care Conversations leading to the ordering of a Goals of Care Designation (GCD) should take place as early as possible in a patient’s course of care. The Goals of Care Designation is created, or the previous GCD -

The Measurement of Serum Osmolality and Its Application to Clinical
Jornal Brasileiro de Patologia e Medicina Laboratorial ISSN: 1676-2444 [email protected] Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial Brasil Faria, Daniel K.; Mendes, Maria Elizabete; Sumita, Nairo M. The measurement of serum osmolality and its application to clinical practice and laboratory: literature review Jornal Brasileiro de Patologia e Medicina Laboratorial, vol. 53, núm. 1, febrero, 2017, pp. 38-45 Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial Rio de Janeiro, Brasil Available in: http://www.redalyc.org/articulo.oa?id=393549993007 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative REVIEW ARTICLE J Bras Patol Med Lab, v. 53, n. 1, p. 38-45, February 2017 The measurement of serum osmolality and its application to clinical practice and laboratory: literature review 10.5935/1676-2444.20170008 A medida da osmolalidade sérica e sua aplicação na prática clínica e laboratorial: revisão da literatura Daniel K. Faria; Maria Elizabete Mendes; Nairo M. Sumita Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, Brazil. ABSTRACT Introduction: Serum osmolality is an essential laboratory parameter to understand several clinical disorders such as electrolyte disturbances, exogenous intoxication and hydration status. Objective: This study aims to update knowledge about the osmolality examination through research papers published to date. Materials and methods: The survey was conducted on PubMed database. It highlights main concepts, both historical and physiological aspects, and the clinical applications of the serum osmolality test. -

109: Toxic Alcohols
109: Toxic Alcohols Sage W. Wiener HISTORY AND EPIDEMIOLOGY Methanol was a component of the embalming fluid used in ancient Egypt. Robert Boyle first isolated the molecule in 1661 by distilling boxwood, calling it spirit of box.29 The molecular composition was determined in 1834 by Dumas and Peligot, who coined the term “methylene” from the Greek roots for “wood wine.”202 Industrial production began in 1923, and today most methanol is used for the synthesis of other chemicals. Methanol containing consumer products that are commonly encountered include model airplane and model car fuel, windshield washer fluid, solid cooking fuel for camping and chafing dishes, photocopying fluid, colognes and perfumes, and gas line antifreeze (“dry gas”). Methanol is also used as a solvent by itself or as an adulterant in “denatured” alcohol.138Most reported cases of methanol poisoning in the United States involve ingestions of one of the above products, with more than 60% involving windshield washer fluid,58 although most inhalational exposures involve carburetor cleaner.87 In a Tunisian series, ingested cologne was the most common etiology.30 In a Turkish series, cologne was also most common, accounting for almost 75% of ingestions.129 Perfume was one of several exposures in a patient with methanol poisoning in a report from Spain,173 and methanol poisoning from cologne has also been reported in India.12 There are sporadic epidemics of mass methanol poisoning, most commonly involving tainted fermented beverages.23,130These epidemics are a continuing problem in many parts of the world.16,146,153,166,187,218,257 Ethylene glycol was first synthesized in 1859 by Charles-Adolphe Wurtz and first widely produced as an engine coolant during World War II, when its precursor ethylene oxide became readily available.70 Today its primary use remains as an engine coolant (antifreeze) in car radiators. -

Ethylene Glycol and Propylene Glycol Toxicity
Agency for Toxic Substances and Disease Registry Case Studies in Environmental Medicine (CSEM) Ethylene Glycol and Propylene Glycol Toxicity Course: WB 4342 CE Original Date: March 20, 2020 CE Expiration Date: March 20, 2022 Key • Ethylene glycol ingestion first affects the central Concepts nervous system (CNS). After a characteristic latent period, toxic metabolites might produce signs of inebriation followed by serious illness and even death. • No studies were located regarding a link between ethylene glycol exposure and cancer or reproductive or developmental hazards in humans. • Propylene glycol is much less toxic than ethylene glycol. About This This educational case study is one in a series of self- and Other instructional modules designed to increase the primary Case Studies care provider’s knowledge of hazardous substances in the in environment. The modules also promote adoption of Environment medical practices that aid in the evaluation and care of al Medicine potentially exposed patients. You can access the complete series of Case Studies in Environmental Medicine on the Agency for Toxic Substances and Disease Registry (ATSDR) website at URL: https://www.atsdr.cdc.gov/emes/health_professionals/ind ex.html. A downloadable PDF version of this educational series and other environmental medicine materials provides content in an electronic, printable format, especially for those who might lack adequate Internet service. Page 1 of 124 Acknowledgments We gratefully acknowledge the work of the medical writers, editors, and reviewers in producing this educational resource. Listed below are the contributors to this version of the Case Study in Environmental Medicine. Please Note: Each content expert for this case study has indicated that he or she has no conflict of interest to disclose that would bias the case study content. -

Methanol Intoxication: the Importance of Early Diagnosis Case Reports
b Meta olis g m OPEN ACCESS Freely available online u & r D T o f x o i l c a o Journal of n l o r g u y o J ISSN: 2157-7609 Drug Metabolism & Toxicology Case Report Methanol Intoxication: The Importance of Early Diagnosis Case Reports and Literature Review of Methanol Intoxication´s Diagnosis and Treatment Beñat de Alba Iriarte1*, Noelia López Barba1, Eztitxu Gaztelumendi Eguiguren2, Felix Zubia Olascoaga3, María Asunción Vives Almandoz1, Eva Lorea Gil Rodríguez1, Miren Arantza Aguillo García2, Jesús Barado Hualde1 1Osakidetza Basque Health Service, Donostia University Hospital, Clinical Biochemistry Laboratory, Donostia-San Sebastián, Spain; 2Osakidetza Basque Health Service, Donostia University Hospital, Emergency Department, Donostia-San Sebastián, Spain; 3Osakidetza Basque Health Service, Donostia University Hospital, Intensive Care Unit, Donostia-San Sebastián, Spain ABSTRACT Methanol intoxication is an unfrequent condition associated with high morbidity that can cause severe metabolic disturbances, blindness, and important neurological dysfunction potentially life-threatening. Therefore, early diagnosis and treatment is required to obtain the best possible result for the patient. Recently, an increase in cases of methanol intoxication has been observed in our environment (50% in the last year) and in our hospital, as in most of them, specific analysis techniques for methanol are not available. That is why in this article we present four recent clinical cases that occurred in our hospital: to analyze the most relevant information derived from the management of these patients and their clinical and laboratory data. Our objective is to develop a valid strategy in order to facilitate obtaining an early diagnosis sometimes difficult to achieve. -

Medical Toxicology Rotation
UCSD Medical Toxicology Please place your name here: __________________ Date of Clerkship:________________________ Please Circle Who You Are: UCSD EM, Navy EM, UCI EM, Rady Peds EM, UCSD IM, UCSD Peds, UCSD Med Student, Outside Resident, Outside Med Student Medical Toxicology Rotation Arranging the Rotation For medical students please contact Jessica Ramirez ([email protected]) (619-543-6463) who is the medical student rotation coordinator. For residents and pediatric fellows please contact Maeve-Anne (Mae) Malong ([email protected]) (619-543-4627) who is the resident and fellow rotation coordinator. Medical Toxicology Rotation Welcome to the Medical Toxicology Rotation. We are happy to have you rotate through and are committed to teaching you as much as we can while you are here. Included in this packet is a guide for the rotation and also a worksheet with checklists and questions that you will need to return (by email to Dr. Schneir) at the end of the rotation. ___ABOUT A WEEK BEFORE YOU BEGIN THE ROTATION PLEASE EMAIL Dr. Schneir at [email protected] TO LET HIM KNOW WHEN YOU START SO HE CAN ARRANGE YOU TO BE ASSIGNED A JOURNAL CLUB ARTICLE AND LET YOU KNOW WHERE TO MEET ON YOUR FIRST DAY Clerkship Director: Aaron Schneir M.D. Office: 858-715-6308 Cell Phone: 619-733-7315 [email protected] Other Full time UCSD Staff: Lee Cantrall Pharm D. Managing Director, San Diego Poison Control Center Cell Phone: 619-733-9570 [email protected] UCSD Medical Toxicology Richard Clark M.D. Chief, Division of Medical Toxicology Cell Phone: 619-733-7310 To reach on call: cell phone only Pager: 800-900-1671 [email protected] Allyson Kreshak M.D.