Strain and Stability
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Conformations of Cycloalkanes
The Conformations of Cycloalkanes Ring-containing structures are a common occurrence in organic chemistry. We must, therefore spend some time studying the special characteristics of the parent cycloalkanes. Cyclical connectivity imposes constraints on the range of motion that the atoms in rings can undergo. Cyclic molecules are thus more rigid than linear or branched alkanes because cyclic structures have fewer internal degrees of freedom (that is, the motion of one atom greatly influences the motion of the others when they are connected in a ring). In this lesson we will examine structures of the common ring structures found in organic chemistry. The first four cycloalkanes are shown below. cyclopropane cyclobutane cyclopentane cyclohexane The amount of energy stored in a strained ring is estimated by comparing the experimental heat of formation to the calculated heat of formation. The calculated heat of formation is based on the notion that, in the absence of strain, each –CH2– group contributes equally to the heat of formation, in line with the behavior found for the acyclic alkanes (i.e., open chains). Thus, the calculated heat of formation varies linearly with the number of carbon atoms in the ring. Except for the 6-membered ring, the experimental values are found to have a more positive heat of formation than the calculated value owning to ring strain. The plots of calculated and experimental enthalpies of formation and their difference (i.e., ring strain) are seen in the Figure. The 3-membered ring has about 27 kcal/mol of strain. It can be seen that the 6-membered ring possesses almost no ring strain. -

Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis Of
The Astrophysical Journal, 889:3 (26pp), 2020 January 20 https://doi.org/10.3847/1538-4357/ab616c © 2020. The American Astronomical Society. All rights reserved. Implications for Extraterrestrial Hydrocarbon Chemistry: Analysis of Acetylene (C2H2) and D2-acetylene (C2D2) Ices Exposed to Ionizing Radiation via Ultraviolet–Visible Spectroscopy, Infrared Spectroscopy, and Reflectron Time-of-flight Mass Spectrometry Matthew J. Abplanalp1,2 and Ralf I. Kaiser1,2 1 W. M. Keck Research Laboratory in Astrochemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA 2 Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA Received 2019 October 4; revised 2019 December 7; accepted 2019 December 10; published 2020 January 20 Abstract The processing of the simple hydrocarbon ice, acetylene (C2H2/C2D2), via energetic electrons, thus simulating the processes in the track of galactic cosmic-ray particles penetrating solid matter, was carried out in an ultrahigh vacuum surface apparatus. The chemical evolution of the ices was monitored online and in situ utilizing Fourier transform infrared spectroscopy (FTIR) and ultraviolet–visible spectroscopy and, during temperature programmed desorption, via a quadrupole mass spectrometer with an electron impact ionization source (EI-QMS) and a reflectron time-of-flight mass spectrometer utilizing single-photon photoionization (SPI-ReTOF-MS) along with resonance-enhanced multiphoton photoionization (REMPI-ReTOF-MS). The confirmation of previous in situ studies of ethylene ice irradiation -

Comparing Models for Measuring Ring Strain of Common Cycloalkanes
The Corinthian Volume 6 Article 4 2004 Comparing Models for Measuring Ring Strain of Common Cycloalkanes Brad A. Hobbs Georgia College Follow this and additional works at: https://kb.gcsu.edu/thecorinthian Part of the Chemistry Commons Recommended Citation Hobbs, Brad A. (2004) "Comparing Models for Measuring Ring Strain of Common Cycloalkanes," The Corinthian: Vol. 6 , Article 4. Available at: https://kb.gcsu.edu/thecorinthian/vol6/iss1/4 This Article is brought to you for free and open access by the Undergraduate Research at Knowledge Box. It has been accepted for inclusion in The Corinthian by an authorized editor of Knowledge Box. Campring Models for Measuring Ring Strain of Common Cycloalkanes Comparing Models for Measuring R..ing Strain of Common Cycloalkanes Brad A. Hobbs Dr. Kenneth C. McGill Chemistry Major Faculty Sponsor Introduction The number of carbon atoms bonded in the ring of a cycloalkane has a large effect on its energy. A molecule's energy has a vast impact on its stability. Determining the most stable form of a molecule is a usefol technique in the world of chemistry. One of the major factors that influ ence the energy (stability) of cycloalkanes is the molecule's ring strain. Ring strain is normally viewed as being directly proportional to the insta bility of a molecule. It is defined as a type of potential energy within the cyclic molecule, and is determined by the level of "strain" between the bonds of cycloalkanes. For example, propane has tl1e highest ring strain of all cycloalkanes. Each of propane's carbon atoms is sp3-hybridized. -

Text Related to Segment 5.02 ©2002 Claude E. Wintner from the Previous Segment We Have the Value of the Heat of Combustion Of
Text Related to Segment 5.02 ©2002 Claude E. Wintner From the previous segment we have the value of the heat of combustion of an "unstrained" methylene unit as -157.4 kcal/mole. On this basis one would predict that combustion of "unstrained" cyclopropane should liberate 3 X 157.4 = 472.2 kcal/mole; however, when cyclopropane is burned, a value of 499.8 kcal/mole is measured for the actual heat of combustion. The difference of 27.6 kcal/mole is interpreted as representing the higher internal energy ("strain energy") of real cyclopropane as compared to a postulated strain-free "model." ("Model" in this usage speaks of a proposal, or postulate.) These facts are graphed conveniently on an energy diagram as follows: "Strain Energy" represents the error units: kcal/mole not to scale in the "strain-free model" real cyclopropane + 4.5 O2 27.6 "strain-free model" of cyclopropane 499.8 observed heat of combustion + 4.5 O2 472.2 3CO2 + 3H2O heat of combustion predicted for "strain-free model" Note that this estimate of the strain energy in cyclopropane amounts to 9.2 kcal/mole of strain per methylene group (dividing 27.6 by 3, because of the three carbon atoms). Comparable values in kcal/mole for other small and medium rings are given in the following table. As one might expect, in general the strain decreases as the ring is enlarged. units: kcal/mole n Total Strain Strain per CH2 3 27.6 9.2 (CH2)n 4 26.3 6.6 5 6.2 1.2 6 0.1 0.0 ! 7 6.2 0.9 8 9.7 1.2 9 12.6 1.4 10 12.4 1.2 12 4.1 0.3 15 1.9 0.1 Without entering into a discussion of the relevant bonding concepts here, and instead relying on geometry alone, interpretation of the source of the strain energy in cyclopropane and cyclobutane is to some extent self-evident. -

Today's Class Will Focus on the Conformations and Energies of Cycloalkanes
Name ______________________________________ Today's class will focus on the conformations and energies of cycloalkanes. Section 6.4 of the text provides an introduction. 1 Make a model of cyclopentane. Use the 2-piece "lockable" bond connectors — these rotate more easily than the smaller ones. You may need to twist them to "lock" so that they don't pop apart. The text shows two conformations of this ring — the envelope and the half-chair. Why is cyclopentane non-planar? The two conformations are below. In the envelope, 4 Cs are in a plane; in the half-chair 3 Cs are in a plane. Th first conformer is easy. To get your model to look like the second one, start with a planar ring, keep the "front" 3 Cs in the plane, and twist the "back" C–C bond so that one C goes above that plane and the other goes below. Add Hs to each of these structures — no wedges and dashes here — these are "perspective" drawings (like the drawings of chair cyclohexane rings on p 197). Just use your model and sketch the Hs where they appear to be. fast If we were to replace one H in each of these conformers with a methyl group, what do you think would be the most favorable position for it? Circle the one H on each structure above that you would replace with a CH3. In general, what happens to the ring C–C–C angles when a ring goes from planar to non-planar? Lecture outline Structures of cycloalkanes — C–C–C angles if planar: 60° 90° 108° 120° actual struct: planar |— slightly |— substantially non-planar —| non-planar —> A compound is strained if it is destabilized by: abnormal bond angles — van der Waals repulsions — eclipsing along σ-bonds — A molecule's strain energy (SE) is the difference between the enthalpy of formation of the compound of interest and that of a hypothetical strain-free compound that has the same atoms connected in exactly the same way. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Are the Most Well Studied of All Ring Systems. They Have a Limited Number Of, Almost Strain Free, Conformations
Chem 201/Beauchamp Topic 6, Conformations (cyclohexanes) 1 Cyclohexanes Cyclohexane rings (six atom rings in general) are the most well studied of all ring systems. They have a limited number of, almost strain free, conformations. Because of their well defined conformational shapes, they are frequently used to study effects of orientation or steric effects when studying chemical reactions. Additionally, six atom rings are the most commonly encountered rings in nature. Cyclohexane structures do not choose to be flat. Slight twists at each carbon atom allow cyclohexane rings to assume much more comfortable conformations, which we call chair conformations. (Chairs even sound comfortable.) The chair has an up and down shape all around the ring, sort of like the zig-zag shape seen in straight chains (...time for models!). C C C C C C lounge chair - used to kick back and relax while you study your organic chemistry chair conformation Cyclohexane rings are flexible and easily allow partial rotations (twists) about the C-C single bonds. There is minimal angle strain since each carbon can approximately accommodate the 109o of the tetrahedral shape. Torsional strain energy is minimized in chair conformations since all groups are staggered relative to one another. This is easily seen in a Newman projection perspective. An added new twist to our Newman projections is a side-by-side view of parallel single bonds. If you look carefully at the structure above or use your model, you should be able to see that a parallel relationship exists for all C-C bonds across the ring from one another. -

Thermodynamic Potential of Free Energy for Thermo-Elastic-Plastic Body
Continuum Mech. Thermodyn. (2018) 30:221–232 https://doi.org/10.1007/s00161-017-0597-3 ORIGINAL ARTICLE Z. Sloderbach´ · J. Paja˛k Thermodynamic potential of free energy for thermo-elastic-plastic body Received: 22 January 2017 / Accepted: 11 September 2017 / Published online: 22 September 2017 © The Author(s) 2017. This article is an open access publication Abstract The procedure of derivation of thermodynamic potential of free energy (Helmholtz free energy) for a thermo-elastic-plastic body is presented. This procedure concerns a special thermodynamic model of a thermo-elastic-plastic body with isotropic hardening characteristics. The classical thermodynamics of irre- versible processes for material characterized by macroscopic internal parameters is used in the derivation. Thermodynamic potential of free energy may be used for practical determination of the level of stored energy accumulated in material during plastic processing applied, e.g., for industry components and other machinery parts received by plastic deformation processing. In this paper the stored energy for the simple stretching of austenitic steel will be presented. Keywords Free energy · Thermo-elastic-plastic body · Enthalpy · Exergy · Stored energy of plastic deformations · Mechanical energy dissipation · Thermodynamic reference state 1 Introduction The derivation of thermodynamic potential of free energy for a model of the thermo-elastic-plastic body with the isotropic hardening is presented. The additive form of the thermodynamic potential is assumed, see, e.g., [1–4]. Such assumption causes that the influence of plastic strains on thermo-elastic properties of the bodies is not taken into account, meaning the lack of “elastic–plastic” coupling effects, see Sloderbach´ [4–10]. -

Physical Organic Chemistry
PHYSICAL ORGANIC CHEMISTRY Yu-Tai Tao (陶雨台) Tel: (02)27898580 E-mail: [email protected] Website:http://www.sinica.edu.tw/~ytt Textbook: “Perspective on Structure and Mechanism in Organic Chemistry” by F. A. Corroll, 1998, Brooks/Cole Publishing Company References: 1. “Modern Physical Organic Chemistry” by E. V. Anslyn and D. A. Dougherty, 2005, University Science Books. Grading: One midterm (45%) one final exam (45%) and 4 quizzes (10%) homeworks Chap.1 Review of Concepts in Organic Chemistry § Quantum number and atomic orbitals Atomic orbital wavefunctions are associated with four quantum numbers: principle q. n. (n=1,2,3), azimuthal q.n. (m= 0,1,2,3 or s,p,d,f,..magnetic q. n. (for p, -1, 0, 1; for d, -2, -1, 0, 1, 2. electron spin q. n. =1/2, -1/2. § Molecular dimensions Atomic radius ionic radius, ri:size of electron cloud around an ion. covalent radius, rc:half of the distance between two atoms of same element bond to each other. van der Waal radius, rvdw:the effective size of atomic cloud around a covalently bonded atoms. - Cl Cl2 CH3Cl Bond length measures the distance between nucleus (or the local centers of electron density). Bond angle measures the angle between lines connecting different nucleus. Molecular volume and surface area can be the sum of atomic volume (or group volume) and surface area. Principle of additivity (group increment) Physical basis of additivity law: the forces between atoms in the same molecule or different molecules are very “short range”. Theoretical determination of molecular size:depending on the boundary condition. -

Klein 4A Alkanes+Isomerism
Grossman, CHE 230 4a. Alkanes. Conformational Stereoisomerism. Structural Isomerism. 4a.1 Derivatives of methane. We can replace one of the H atoms in methane with another atom or group. These atoms or groups are called heteroatoms. Examples: Methyl bromide, methyl iodide, methanol, methylamine, methanethiol. Here the central C atom is not quite as perfectly tetrahedral, but for our purposes, it is close enough. If the H atom is replaced with nothing, we have three possibilities. – • We can remove just the H nucleus and leave behind two electrons -- this gives CH3 , with sp3 hybridization. It has the same structure as ammonia, just one fewer proton (and neutron) in the nucleus in the center. + • We can take away the H nucleus and both electrons. This gives us CH3 . The hybridization here? We don't want to waste valuable low-energy s orbital in a hybrid orbital in which there are no electrons. Instead, the valuable s electron is divided equally among the six electrons (three bonds) remaining. Three bonds means three orbitals, one s and two p. That means the third p orbital remains unhybridized. This situation is called sp2 hybridization. Two p orbitals occupy a plane, so in sp2 hybridization, the three hybrid orbitals are coplanar. They point 120° apart, to the corners of a triangle. The unhybridized p orbital is perpendicular to this plane. • What if we take away the H nucleus and one electron? We have a situation in which CH3· (methyl radical) is between sp2 and sp3 hybridization. It's a shallow pyramid. These species are high in energy and don't exist for long. -

Chem 341 • Organic Chemistry I Lecture Summary 10 • September 14, 2007
Chem 341 • Organic Chemistry I Lecture Summary 10 • September 14, 2007 Chapter 4 - Stereochemistry of Alkanes and Cycloalkanes Conformations of Cycloalkanes Cyclic compounds contain something we call Ring Strain. There are three things that contribute to ring strain. Torsional strain (electron repulsion in eclipsing bonds), steric strain (atoms bumping into each other) and angle strain. Angle Strain: the strain due to bond angles being forced to expand or contract from their ideal. Sp3 hybridized atoms want to have bond angles of 109.5°. However, if the rings are very small or very large, there is no way to accommodate this angle. Thus, this increases the energy of the molecule. Heat of Combustion: the amount of heat (energy) released when a molecule burns completely with oxygen. By comparing the heat of combustion of different sized cycloalkanes, their relative energies can be obtained. The fact that the size of the ring has an influence on the total energy of the molecule indicates that there is some degree of instability associated with constraining the rings. This added energy (in addition to what would be expected from carbon and hydrogen combustion per mole) can be attributed to ring strain. n i a r t S g n i R 3 4 5 6 7 8 9 10 11 12 13 14 Ring Size Conformations of Cyclopropane Cyclopropane has a high degree of angle strain due to the highly distorted bond angles. The angle between the carbons is 60°. This cannot be accommodated by sp3-hybridized atoms. Thus, the 60° bonds actually are bent sigma bonds. -
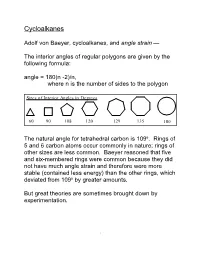
Cycloalkanes
Cycloalkanes Adolf von Baeyer, cycloalkanes, and angle strain — The interior angles of regular polygons are given by the following formula: angle = 180(n -2)/n, where n is the number of sides to the polygon Sizes of Interior Angles in Degrees 60 90 108 120 129 135 180 The natural angle for tetrahedral carbon is 109o. Rings of 5 and 6 carbon atoms occur commonly in nature; rings of other sizes are less common. Baeyer reasoned that five and six-membered rings were common because they did not have much angle strain and therefore were more stable (contained less energy) than the other rings, which deviated from 109o by greater amounts. But great theories are sometimes brought down by experimentation. 1 Let's consider several acyclic alkanes — no rings, therefore, no strain energy. Burn each of them to form carbon dioxide and water and measure the heat of combustion. Compound DH, kcal/mole DDH, kcal/mole propane-C3H8 530.4 157.4 butane-C4H10 687.8 157.2 pentane-C5H12 845.0 157.6 hexane-C6H14 1002.6 Average 157.4 So, the average heat of combustion for a CH2 unit in an unstrained alkane is 157.4 kcal/mole. We might write: n CH2 + n(3/2) O2 -------> n CO2 + n H2O + n(157.4 kcal) Strained rings, which have more energy than unstrained ones, should give off more heat per CH2 unit when they are burned to give carbon dioxide and water. Let's see. 2 (CH2)n DH, kcal/mol DH/CH2, Strain kcal/mol energy/CH2 (CH2)3 499.8 166.6 9.2 (CH2)4 655.9 164.0 6.6 (CH2)5 793.5 158.7 1.3 (CH2)6 944.5 157.4 0 (CH2)7 1108 158.3 0.9 (CH2)8 1269 158.6 1.2 (CH2)9 1429 158.8 1.4 (CH2)10 1586 158.6 1.2 (CH2)11 1742 158.4 1.0 (CH2)12 1891 157.6 0.2 (CH2)13 2051 157.8 0.4 (CH2)14 2204 157.4 0 Bayer was right about the 3- and 4-member rings having angle strain.