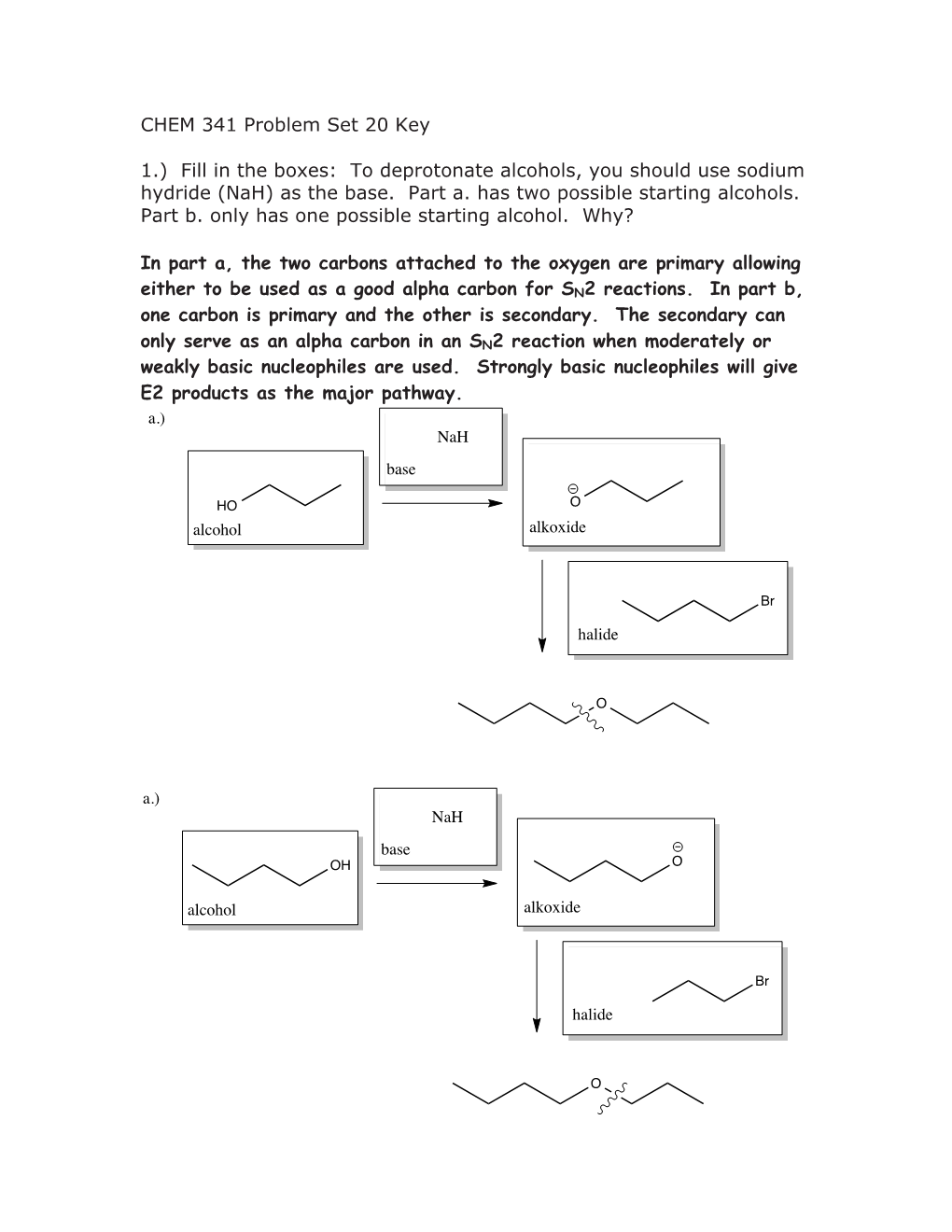
To Deprotonate Alcohols, You Should Use Sodium Hydride (Nah) As the Base
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

23.5 Basicity and Acidity of Amines
23_BRCLoudon_pgs5-0.qxd 12/8/08 1:22 PM Page 1122 1122 CHAPTER 23 • THE CHEMISTRY OF AMINES alkylamines, this resonance occurs at rather small chemical shift—typically around d 1. In aromatic amines, this resonance is at greater chemical shift, as in the second of the preceding examples. Like the OH protons of alcohols, phenols, and carboxylic acids, the NH protons of amines under most conditions undergo rapid exchange (Secs. 13.6 and 13.7D). For this reason, split- ting between the amine N H and adjacent C H groups is usually not observed. Thus, in the NMR spectrum of diethylamine,L the N H resonanceL is a singlet rather than the triplet ex- pected from splitting by the adjacent CHL2 protons. In some amine samples, the N H resonance is broadened and, like the OL H protonL of alcohols, it can be obliterated fromL the spectrum by exchange with D2O (the “DL2O shake,” p. 611). The characteristic 13C NMR absorptions of amines are those of the a-carbons—the carbons attached directly to the nitrogen. These absorptions occur in the d 30–50 chemical-shift range. As expected from the relative electronegativities of oxygen and nitrogen, these shifts are somewhat less than the a-carbon shifts of ethers. PROBLEMS 23.4 Identify the compound that has an M 1 ion at mÜz 136 in its CI mass spectrum, an IR 1 + = absorption at 3279 cm_ , and the following NMR spectrum: d 0.91 (1H, s), d 1.07 (3H, t, J 7Hz), d 2.60 (2H, q, J 7Hz), d 3.70 (2H, s), d 7.18 (5H, apparent s). -

S.T.E.T.Women's College, Mannargudi Semester Iii Ii M
S.T.E.T.WOMEN’S COLLEGE, MANNARGUDI SEMESTER III II M.Sc., CHEMISTRY ORGANIC CHEMISTRY - II – P16CH31 UNIT I Aliphatic nucleophilic substitution – mechanisms – SN1, SN2, SNi – ion-pair in SN1 mechanisms – neighbouring group participation, non-classical carbocations – substitutions at allylic and vinylic carbons. Reactivity – effect of structure, nucleophile, leaving group and stereochemical factors – correlation of structure with reactivity – solvent effects – rearrangements involving carbocations – Wagner-Meerwein and dienone-phenol rearrangements. Aromatic nucleophilic substitutions – SN1, SNAr, Benzyne mechanism – reactivity orientation – Ullmann, Sandmeyer and Chichibabin reaction – rearrangements involving nucleophilic substitution – Stevens – Sommelet Hauser and von-Richter rearrangements. NUCLEOPHILIC SUBSTITUTION Mechanism of Aliphatic Nucleophilic Substitution. Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. For that reason, it attracts to nucleophile In organic chemistry and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which a leaving group(nucleophile) is replaced by an electron rich compound(nucleophile). The whole molecular entity of which the electrophile and the leaving group are part is usually called the substrate. The nucleophile essentially attempts to replace the leaving group as the primary substituent in the reaction itself, as a part of another molecule. The most general form of the reaction may be given as the following: Nuc: + R-LG → R-Nuc + LG: The electron pair (:) from the nucleophile(Nuc) attacks the substrate (R-LG) forming a new 1 bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. -

Synthesis and Chemistry of Indole
Synthesis and Chemistry of Indole 1. Introduction: ➢ Indole is a benzo[b]pyrrole formed by the fusion of benzene ring to the 2,3 positions of pyrrole nucleus. ➢ The word “Indole” is derived from the word India, as the heterocycle was first isolated from a blue dye “Indigo” produced in India during sixteenth century. ➢ In 1886, Adolf Baeyer isolated Indole by the pyrolysis of oxindole with Zn dust. Oxindole was originally obtained by the reduction of isatin, which in turn was isolated by the oxidation of Indigo. ➢ Commercially indole is produced from coal tar ➢ Indole is the most widely distributed heterocyle ➢ Indole nucleus is an integral part of thousands of naturally occurring alkaloids, drugs and other compounds. By Dr. Divya Kushwaha 2. Synthesis of Indole 2.1. Fischer Indole Synthesis: ➢ This reaction was discovered in 1983 by Emil Fischer and so far remained the most extensively used method of preparation of indoles. ➢ The synthesis involves cyclization of arylhydrazones under heating conditions in presence of protic acid or lewis acids such as ZnCl2, PCl3, FeCl3, TsOH, HCl, H2SO4, PPA etc. ➢ The starting material arylhydrazoles can be obtained from aldehydes, ketones, keto acids, keto esters and diketones etc. ➢ Reaction produces 2,3-disubtituted products. Unsymmetrical ketones can give a mixture of indoles. 2.2 Madelung Synthesis: ➢ Base catalyzed cyclization of 2-(acylamino)-toluenes under very harsh conditions (typically sodium amide or potassium t-butoxide at 250-300oC). ➢ Limited to the synthesis of simple indoles such as 2-methyl indoles without having any sensitive groups. By Dr. Divya Kushwaha Mechanism: ➢ A modern variant of madelung reaction is performed under milder conditions by the use of alkyllithiums as bases. -

Chem 232 Lecture 19
CHEM 232 University of Illinois Organic Chemistry I at Chicago UIC Lecture 19 Organic Chemistry 1 Professor Duncan Wardrop March 16, 2010 1 CHEM 232 University of Illinois Organic Chemistry I at Chicago UIC Chapter 9 Alkynes Sources and Nomenclature Sections 9.1 - 9.6 2 CHEM 232 University of Illinois Organic Chemistry I at Chicago UIC Exam Two • Monday, April 5 • 6:00-7:15 p.m. • 250 SES • Chapters 6-10, 13 • Makeup Exam: Monday, April 12, time t.b.a. Makeup policy: There are no makeup exams without prior approval. Only students showing proof of a class con!ict will have the option to take a makeup exam. To be added to the makeup list, you must email me no later than Friday, April. 2. 3 Self-Test Question Which column lists the correct mechanistic symbol for each description? A B C D E Methyl halides react with sodium ethoxide in ethanol only by this mechanism. SN2 SN1 E2 SN2 SN1 Unhindered primary halides react with sodium ethoxide in ethanol mainly by this mechanism. SN2 SN2 SN2 SN2 SN1 Cyclohexyl bromide reacts with sodium ethoxide in ethanol, mainly by this mechanism. E2 SN1 E2 SN2 SN1 The product obtained by solvolysis of tert-butyl bromide in ethanol arises by this mechanism. SN1 SN2 SN2 SN2 E2 In ethanol that contains sodium ethoxide, tert-butyl bromide in ethanol reacts mainly by this mechanism. E2 E2 SN1 SN1 E2 University of Slide CHEM 232, Spring 2010 4 Illinois at Chicago UIC Lecture 19: March 16 4 Substitution/Elimination Review SN2 H3C Cl + OCH2CH3 H3C OCH2CH3 Methyl halides react with sodium ethoxide in • methyl halide = ethanol only by this mechanism. -

Ethylamine Derivatives and Their Use As Antihypertensive Agents
Europaisches Patentamt European Patent Office (fl) Publication number: 0 294 183 Office europeen des brevets A1 ® EUROPEAN PATENT APPLICATION @ Application number: 88305012.2 ® int. a*: C 07 D 211/32 C 07 D 211/22, @ Date of filing: 01.06.88 C 07 D 211/18, C 07 D 285/24, C 07 D 401/06, A 61 K 31/445, A 61 K 31/54 (So) Priority: 02.06.87 JP 138405/87 Toyota, Kozo No. 1-1 Suzuki-cho Kawasaki-ku @ Date of publication of application : Kawasaki-shi Kanagawa-ken (JP) 07.12.88 Bulletin 88/49 Yoshimoto, Ryota No. 1-1 Suzuki-cho Kawasaki-ku @ Designated Contracting States: Kawasaki-shi Kanagawa-ken (JP) CH DE FR GB IT LI Koyama, Yosikatsu @ Applicant: AJINOMOTO CO., INC. No. 1-1 Suzuki-cho Kawasaki-ku 5-8, Kyobashi 1-chome, Chuo-ku Kawasaki-shi Kanagawa-ken (JP) Tokyo 104 (JP) Domoto, Hideki No. 1-1 Suzuki-cho Kawasaki-ku @ Inventor: Syoji, Masataka Kawasaki-shi Kanagawa-ken (JP) No. 1-1 Suzuki-cho Kawasaki-ku Kawasaki-shi Kanagawa-ken (JP) Kamimura, Akira No. 1-1 Suzuki-cho Kawasaki-ku Eguchi, Chikahiko Kawasaki-shi Kahagawa-ken (JP) No. 1-1 Suzuki-cho Kawasaki-ku Kawasaki-shi Kanagawa-ken (JP) Representative: Armitage, Ian Michael et al MEWBURN ELLIS & CO. 2/3 Cursitor Street London EC4A1BQ (GB) (54) Ethylamine derivatives and their use as antihypertensive agents. @ Novel ethylamine derivatives of the formula (I) and salts thereof are useful as antihypertensive agents. Q-X N CH2CH2-A-B (I) \ / CH^ CO 00 wherein A is a linking group wherein the linkage is formed by a carbon or nitrogen atom; B is an aryl-containing group; C is O) hydrogen or optionally substituted alkyl, aralkyl or aryl, or C may CM be bonded to A to form an optionally substituted alkylene bridge; Q is an aryl- or heterocyclic aryl-containing group; X is a linking group wherein the linkage is formed by optionally CL substituted alkylene having from 2 to 20 carbon atoms and/or HI one or more hetero atoms selected from nitrogen and sulfur. -

Aqueous Acid–Base Equilibriums”, Chapter 16 from the Book Principles of General Chemistry (Index.Html) (V
This is “Aqueous Acid–Base Equilibriums”, chapter 16 from the book Principles of General Chemistry (index.html) (v. 1.0M). This book is licensed under a Creative Commons by-nc-sa 3.0 (http://creativecommons.org/licenses/by-nc-sa/ 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz (http://lardbucket.org) in an effort to preserve the availability of this book. Normally, the author and publisher would be credited here. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Additionally, per the publisher's request, their name has been removed in some passages. More information is available on this project's attribution page (http://2012books.lardbucket.org/attribution.html?utm_source=header). For more information on the source of this book, or why it is available for free, please see the project's home page (http://2012books.lardbucket.org/). You can browse or download additional books there. i Chapter 16 Aqueous Acid–Base Equilibriums Many vital chemical and physical processes take place exclusively in aqueous solution, including the complex biochemical reactions that occur in living organisms and the reactions that rust and corrode steel objects, such as bridges, ships, and automobiles. Among the most important reactions in aqueous solution are those that can be categorized as acid–base, precipitation, and complexation reactions. -

The Action of Sodiumamide on Pyridine, and Some Properties of Α
Chemistry. - "Tlte Action o} Sodiumamide on P.'l/1·idine, and some Properties of a-arninopyridine". By J. P . WIBAUT and ELISABETH DINGEMANS~; . (Communicated by Pmf. A. F . HOLJ,)t;MAN). (Communicated at the meetiD~ of December 30, 1922). Through TSCHITSCHIBABIN' S 1) beautiful researches a-aminopyridine has , become easily accessible. This investigator found tllat sodium amide acts on pyridine as follows: C,H,N + NaNH~ = C,H.N.NHNa + H,. On decomp?sition of lhe reartion p"oduct with water, amillopyridine 8,nd sodium hydroxide is formed. As we requil'ed tlli s substauce a s startjng ma.terial for !lynthetie investigations, we have applied the method of pl'epnration found by TSCHITSCHIBABIN. Though also in oU!' experiments a-arninopyridine was fOl'llled as ehief Pl'oduet, we found other substances than the Russian investigatol' among t.he by-pl'oducts. We experienced thaI. the action of sodium amide on pyddine can take place in different ways, dependent 011 th~ nature of the sodium amide preparation used. We ha\'e prepal'ed sodiurn amide according 10 TITm;RLEY'S indiCátion by the action of carefully dried ammonia on melted sodium at 350-400° C. The prepamtion obtained was a pure white, showed a cryst.alline fracture, and contained no free sodium. This preparation did not reaet wÎth pyridine. A p"eparation prepared at 300°, reacted very slowly with pyridine. In this expe I'Ïment ve,·y little a-amino pyridine was however, formed; fmther a little y-y-dipY"idyl, and some other products, which we did not examine. -

CH107 Mock Exam 3 Name: 100
CH107 Mock Exam 3 Name: ______________________________ 100 pts. 1-34 2pts, 35-40 8pts. (choose 4 of 6) 1) A lipid is composed of A) Polar and nonpolar parts B) 2 polar heads C) Phosphate and fatty acids D) A long hydrophobic tail E) 2 fatty acids 2) What has the highest melting point? A) Stearic acid (18:0) Saturated fat (all single bonded carbons) has a higher melting point than unsaturated fat (contains 1 or more double bonds between carbons). The longer the carbon chain, the higher the melting point B) Oleic acid (18:1) this annotation means 18 carbon chain, 1 double bond. So this is an unsaturated fat = low melting point. Think vegetable oil (unsaturated fat) vs. butter (saturated fat). 1 is a liquid at room temp and the other is solid at room temp C) Myristic acid 14 carbon saturated fat. 2nd highest melting point out of 5 options D) Linoleic acid unsaturated fat, omega-6. Super low melting point due to multiple double bonds E) Butyric acid very short carbon chain. But = 4 carbons. 3) Lipids allow for the formation of ______ and _______. A) Membranes, lips can form membranes but not lips B) Micelles, bilayers micelles are formed by lipids having a nonpolar, hydrophobic carbon chain tail and a polar, hydrophilic head. The nonpolar tails stick together to avoid H2O while the polar heads act as a barrier and touch the water in solution. This makes a kind of bubble with all the nonpolar tails stuck together and the polar heads around the outside. -
Sodium Amide Sam
SODIUM AMIDE SAM CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: Pure; Technical Common Synonyms Solid Colorless Odorless Flammable solid 7.2 Storage Temperature: Ambient Sodamide 4.2 Flammable Limits in Air: Not pertinent 7.3 Inert Atmosphere: Must be dry 4.3 Fire Extinguishing Agents: Dry soda Sinks and reacts violently with water. 7.4 Venting: Sealed containers must be stored in ash, graphite, salt, or other well-ventilated area. Keep people away. Avoid contact with liquid and vapor. recommended dry powder, such as dry Wear rubber overclothing (including gloves). limestone. 7.5 IMO Pollution Category: Currently not available Shut off ignition sources and call fire department. 4.4 Fire Extinguishing Agents Not to Be 7.6 Ship Type: Currently not available Notify local health and pollution control agencies. Used: Water 7.7 Barge Hull Type: Currently not available Protect water intakes. 4.5 Special Hazards of Combustion Products: Toxic and irritating ammonia Fire FLAMMABLE. gas may be formed. 8. HAZARD CLASSIFICATIONS POISONOUS GAS IS PRODUCED IN FIRE. 4.6 Behavior in Fire: Currently not available 8.1 49 CFR Category: Not listed. DO NOT USE WATER ON FIRE. 4.7 Auto Ignition Temperature: Not pertinent 8.2 49 CFR Class: Not pertinent. Extinguish with dry graphite, soda ash, powdered limestone, or other approved dry powder. 4.8 Electrical Hazards: Currently not 8.3 49 CFR Package Group: Not listed. available 8.4 Marine Pollutant: No Exposure CALL FOR MEDICAL AID. 4.9 Burning Rate: Not pertinent 8.5 NFPA Hazard Classification: 4.10 Adiabatic Flame Temperature: Currently SOLID not available Category Classification Will burn skin and eyes. -

CHEMISTRY 30A Final Exam FALL 2014 LANEY COLLEGE KEY Dr Schaleger
CHEMISTRY 30A Final Exam FALL 2014 LANEY COLLEGE KEY Dr Schaleger 1. (4). What is the formula of the sulfide of lithium? Li2S 2. (4). How many neutrons are present in the nucleus of chlorine-35? 18 3. (5). What is the pH of 0.0010 M NaOH? 11.0. 4. (5). Hydrogen chloride, a gas, dissolves in water with the evolution of a considerable amount of heat. Write a chemical equation that explains this observation. + ─ HCl(g) + H2O(l) H3O + Cl 5. (5). Provide the missing product in the following nuclear reaction: 238 4 234 94Pu 2He + ? Answer: 92U 6. (10). Sodium amide, NaNH2, reacts with water in a Brønsted acid-base reaction as follows: NaNH2(s) + H2O(l) NH3(aq) + NaOH(aq) a. Write the net ionic equation for this reaction. + ─ NaNH2(s) + H2O(l) NH3(aq) + Na (aq) + OH (aq) b. Which is the stronger base, __X__sodium amide or ____sodium hydroxide? 7. (4). What is the oxidation number of chlorine in NaOCl? Answer: +1. 8. (5). The half-life of C-14 is about 6000 years. After 18,000 years, what percentage of the original amount of the isotope would remain? Answer: Three half-lives or 12.5%. 9. (4). Although helium is a gas under normal conditions, it becomes a liquid at about 4 K. The principal force of attraction holding atoms together in its liquid phase is: a. ____Ion-ion b. ____Dipole-dipole c. __X__London dispersion (induced dipole-induced dipole) d. ____Hydrogen bonding Page | 1 CHEMISTRY 30A Final Exam FALL 2014 LANEY COLLEGE KEY Dr Schaleger 10. -

(Rmgx) Problem Set You Will Be Using Grignard Reagents Throughout This Course to Make Carbon-Carbon Bonds
CHEM 341 Problem Set 18 Problem Set 10/27/17 Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. Making Grignard Reagents: Molecules containing a carbon halogen bond can be transformed into a Grignard reagent. Iodides react faster than bromides. Bromides react faster than chlorides. Fluorides react very slowly to form Grignards and are in general not used. Bromides are the most widely used because they balance good reactivity while not as high of cost as iodides. To make a Grignard reagent, simply add magnesium metal and diethyl ether to a molecule containing a carbon halogen bond. The magnesium will insert itself between the carbon halogen bond. (The actual mechanism probably involves unpaired electrons, but I will never ask for that mechanism) Mg R X R MgX O Grignards are highly reactive compounds. First, and foremost, they are REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!! Grignards are essentially carbanions. They will react with acidic protons such as OH’s, NH’s, SH’s, and terminal alkynes. So, the Grignard reagent cannot have an acidic hydrogen on it. Second, they will react with carbon nitrogen or carbon oxygen pi bonds. Grignards cannot have carbonyls or nitriles. They also cannot contain epoxides. Third, they cannot have a leaving group (even a poor one like OMe) attached to the same carbon next to the halogen. Such Grignards eliminate to give alkenes. Examples of Bad Grignard Reagents MgCl MgBr MeO HO MgBr 1.) Circle the following molecules that can be made into a Grignard reagent.