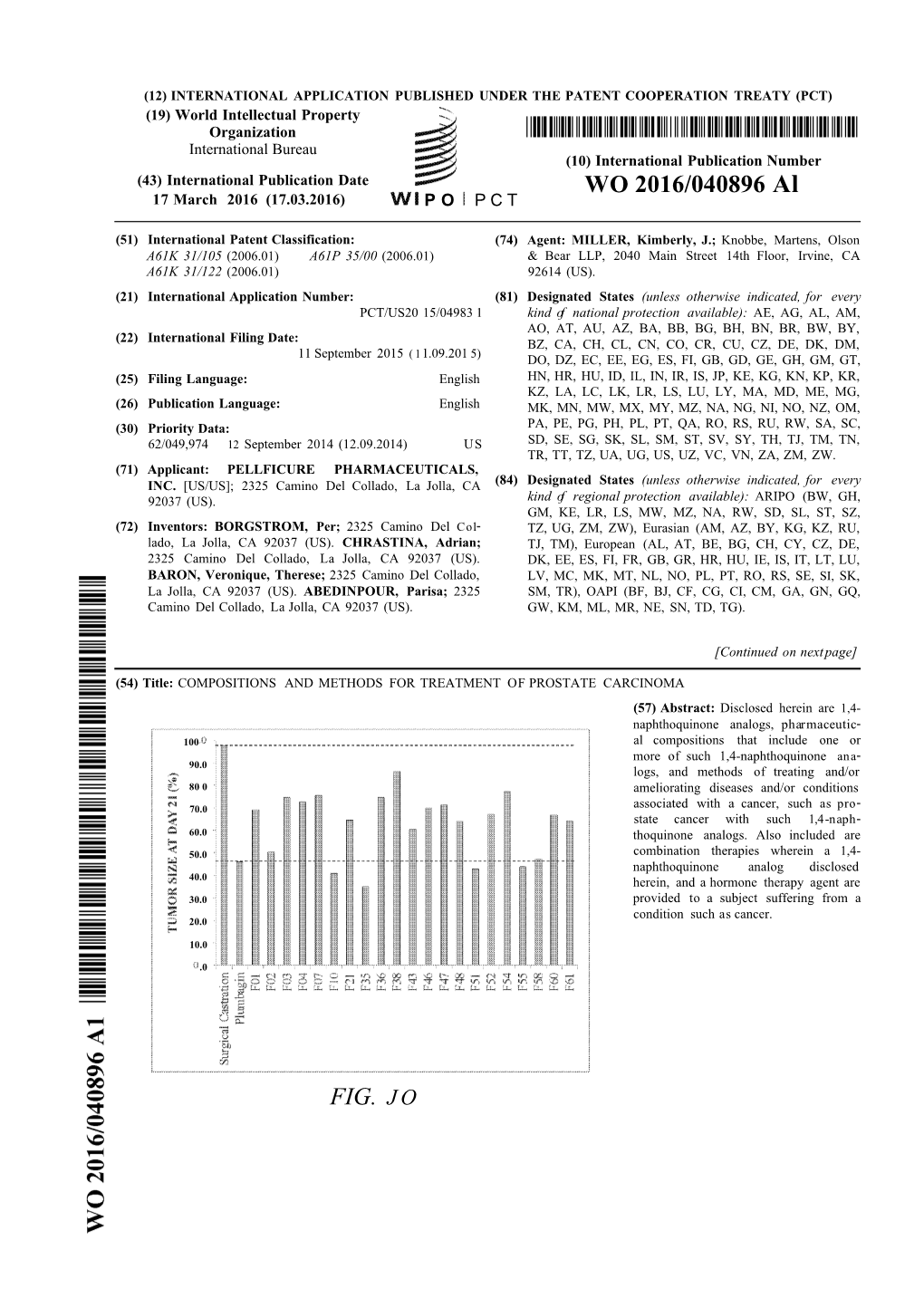
WO 2016/040896 Al FIG. JO
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

Specifications of Approved Drug Compound Library
Annexure-I : Specifications of Approved drug compound library The compounds should be structurally diverse, medicinally active, and cell permeable Compounds should have rich documentation with structure, Target, Activity and IC50 should be known Compounds which are supplied should have been validated by NMR and HPLC to ensure high purity Each compound should be supplied as 10mM solution in DMSO and at least 100µl of each compound should be supplied. Compounds should be supplied in screw capped vial arranged as 96 well plate format. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0221245 A1 Kunin (43) Pub
US 2010O221245A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0221245 A1 Kunin (43) Pub. Date: Sep. 2, 2010 (54) TOPICAL SKIN CARE COMPOSITION Publication Classification (51) Int. Cl. (76) Inventor: Audrey Kunin, Mission Hills, KS A 6LX 39/395 (2006.01) (US) A6II 3L/235 (2006.01) A638/16 (2006.01) Correspondence Address: (52) U.S. Cl. ......................... 424/133.1: 514/533: 514/12 HUSCH BLACKWELL SANDERS LLP (57) ABSTRACT 4801 Main Street, Suite 1000 - KANSAS CITY, MO 64112 (US) The present invention is directed to a topical skin care com position. The composition has the unique ability to treat acne without drying out the user's skin. In particular, the compo (21) Appl. No.: 12/395,251 sition includes a base, an antibacterial agent, at least one anti-inflammatory agent, and at least one antioxidant. The (22) Filed: Feb. 27, 2009 antibacterial agent may be benzoyl peroxide. US 2010/0221 245 A1 Sep. 2, 2010 TOPCAL SKIN CARE COMPOSITION stay of acne treatment since the 1950s. Skin irritation is the most common side effect of benzoyl peroxide and other anti BACKGROUND OF THE INVENTION biotic usage. Some treatments can be severe and can leave the 0001. The present invention generally relates to composi user's skin excessively dry. Excessive use of some acne prod tions and methods for producing topical skin care. Acne Vul ucts may cause redness, dryness of the face, and can actually garis, or acne, is a common skin disease that is prevalent in lead to more acne. Therefore, it would be beneficial to provide teenagers and young adults. -

LHRH) Antagonist Cetrorelix and LHRH Agonist Triptorelin on the Gene Expression of Pituitary LHRH Receptors in Rats
Comparison of mechanisms of action of luteinizing hormone-releasing hormone (LHRH) antagonist cetrorelix and LHRH agonist triptorelin on the gene expression of pituitary LHRH receptors in rats Magdolna Kovacs*†‡ and Andrew V. Schally*†§ *Endocrine, Polypeptide, and Cancer Institute, Veterans Affairs Medical Center, New Orleans, LA 70112; and †Section of Experimental Medicine, Department of Medicine, Tulane University School of Medicine, New Orleans, LA 70112 Contributed by Andrew V. Schally, August 21, 2001 The mechanisms through which luteinizing hormone (LH)-releasing however, are different. LHRH agonists achieve the inhibition of hormone (LHRH) antagonists suppress pituitary gonadotroph func- gonadotropin secretion after a period of continuous exposure (1, tions and LHRH-receptor (LHRH-R) expression are incompletely un- 2, 11–14). In contrast, antagonists of LHRH produce a compet- derstood. Consequently, we investigated the direct effect of LHRH itive blockade of LHRH-R and cause an immediate cessation of antagonist cetrorelix in vitro on the expression of the pituitary the release of gonadotropins and sex steroids, reducing the time LHRH-R gene and its ability to counteract the exogenous LHRH and of the onset of therapeutic effects as compared with the agonists the agonist triptorelin in the regulation of this gene. We also com- (1, 2, 15–17). LHRH agonists such as triptorelin, leuprolide, pared the effects of chronic administration of cetrorelix and triptore- buserelin, or goserelin (1, 2, 14) have been used worldwide for lin on the LHRH-R mRNA level and gonadotropin secretion in ovari- nearly two decades, but LHRH antagonists such as cetrorelix, ectomized (OVX) and normal female rats. The exposure of pituitary ganirelix, and Abarelix have been introduced into the clinical cells in vitro to 3-min pulses of 1 nM LHRH or 0.1 nM triptorelin for 5 h practice relatively recently (1, 2, 15, 16). -

World Journal of Pharmaceutical Research Sonam Et Al
World Journal of Pharmaceutical Research Sonam et al . World Journal of Pharmaceutical SJIF ImpactResearch Factor 5.990 Volume 4, Issue 8, 2393-2402. Review Article ISSN 2277– 7105 5-α-REDUCTASE INHIBITORS - A REVIEW Sonam Kaushal* and Manish Sinha Department of Pharmaceutical Chemistry, ASBASJSM College, Bela, Ropar, India. ABSTRACT Article Received on 19 June 2015, 5-alpha reductase is an important enzyme in metabolic pathway of Revised on 10 July 2015, testosterone. Its three subtypes SRD5A1, SRD5A2 andSRD5A3 are Accepted on 02 Aug 2015 responsible for conversion of testosterone to its more potent derivative dihydrotestosterone (DHT). Excess production of DHT leads to *Correspondence prostate cancer and alopecia. The production of DTH can be reduced For Author by inhibiting the 5-alpha reductase enzyme. Several 5-alpha reductase Sonam Kaushal Department of inhibitors were synthesized in the past and are marketed and used for Pharmaceutical the treatment of prostate cancer and baldness. The dosing of these Chemistry, ASBASJSM drugs is between 1 to 5 mg/day. The low dosing of these drugs need College, Bela, Ropar, special attention on quality control and quantitative estimation of drug India. in formulation and biological fluids. The details of 5-alpha reductase inhibitors and the recent developments in their quantitative estimation have been compiled in the presented review. KEYWORDS: 5-α-reductae inhibitors, cancer, quantitative estimation. 1. INTRODUCTION 1. 5-α-Reductase[1, 2, 3] 1.1. 5-α-reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in 3 metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. -

Personalized ADT
Personalized ADT Thomas Keane MD Conflicts • Ferring • Tolemar • Bayer • Astellas • myriad Personalized ADT for the Specific Paent • Cardiac • OBesity and testosterone • Fsh • High volume metastac disease • Docetaxol • Significant LUTS Cardiovascular risk profile and ADT Is there a difference? Degarelix Belongs to a class of synthe@c drug, GnRH antagonist (Blocker) GnRH pGlu His Trp Ser Tyr Gly Leu Arg Pro Gly NH2 Leuprolide D-Leu NEt Goserelin D-Ser NH2 LHRH agonists Triptorelin D-Trp NH2 Buserelin D-Ser NEt Degarelix D-NaI D-Cpa D-PaI Aph D-Aph D-Ala NH2 N-Me ABarelix D-NaI D-Cpa D-PaI D-Asn Lys D-Ala NH2 Tyr GnRH antagonists Cetrorelix D-NaI D-Cpa D-PaI D-Cit D-Ala NH2 Ganirelix D-NaI D-CPa D-PaI D-hArg D-hArg D-Ala NH2 Millar RP, et al. Endocr Rev 2004;25:235–75 Most acute CVD events are caused By rupture of a vulnerable atherosclero@c plaque The vulnerable plaque – thin cap with inflammaon Inflammation Plaque instability is at the heart of cardiovascular disease Stable plaque Vulnerable plaque Lumen Lumen Lipid core Lipid core FiBrous cap FiBrous cap Thick Cap Thin Rich in SMC and matrix Composion Rich in inflammatory cells: proteoly@c ac@vity Poor Lipid Rich Inflammatory Inflammatory state Highly inflammatory LiBBy P. Circulaon 1995;91:2844-2850 Incidence of Both prostate cancer and CV events is highest in older men Prostate cancer CV events 3500 3500 Prostate cancer All CV disease Major CV events 3000 3000 2827.1 2500 2500 2338.9 2000 2000 1719.7 1500 1500 1152.6 1008.7 1038.7 1000 1000 641.2 545.2 571.1 Age-specific incidence per 100,000 person-years 500 500 246.9 133.7 4.3 0 0 40-49 50-59 60-69 70-79 80-89 90-99 40-49 50-59 60-69 70-79 80-89 90-99 CV, cardiovascular Major CV events = myocardial infarc@on, stroke, or death due to CV disease All CV disease = major CV events + self-reported angina or revascularisaon procedures Driver, et al. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Study Protocol: LPCN 1021-18-001
Oral Testosterone Undecanoate Protocol No. LPCN 1021-18-001 Lipocine Inc. TU, LPCN 1021 675 Arapeen Drive, Suite 202 Salt Lake City, UT-84108 1.0 TITLE PAGE Clinical Study Protocol: LPCN 1021-18-001 Ambulatory Blood Pressure Monitoring in Oral Testosterone Undecanoate (TU, LPCN 1021) Treated Hypogonadal Men. Investigational Product : Testosterone Undecanoate (TU, LPCN 1021) Date of Protocol : 19 February 2019 FDA IND No. : 106476 Development Phase : Phase 3 Testosterone replacement therapy in adult, 18 years or older, males for conditions associated with a deficiency or absence of endogenous Indication : testosterone – primary hypogonadism (congenital or acquired) or secondary hypogonadism (congenital or acquired) Investigator : Multi-Center, US Sponsor : Lipocine Inc. 675 Arapeen Drive, Suite 202, Salt Lake City, Utah – 84108 Tel: +1-801-994-7383 Fax: +1-801-994-7388 Sponsor / : Nachiappan Chidambaram Emergency Contact 675 Arapeen Drive, Suite 202, Salt Lake City, Utah – 84108 Tel: +1-801-994-7383, Ext 2188 Fax: +1-801-994-7388 Email: [email protected] Protocol Version : 06 Confidentiality Statement This document is a confidential communication of Lipocine Inc. Acceptance of this document signifies agreement by the recipient that no unpublished information contained within will be published or disclosed to a third party without prior written approval, except that this document may be disclosed to an Institutional Review Board under the same conditions of confidentiality. Date: 19 February 2019 Confidential Page 1 of 50 Oral Testosterone Undecanoate Protocol No. LPCN 1021-18-001 Lipocine Inc. TU, LPCN 1021 675 Arapeen Drive, Suite 202 Salt Lake City, UT-84108 2.0 SUMMARY OF CHANGES TO PROTOCOL VERSION 2 Version 02 of the LPCN 1021-18-001 study protocol was developed to make the following changes to the study: • Added sexual desire and sexual distress questions to pre-treatment and post-treatment phases of the study. -

(12) United States Patent (10) Patent No.: US 9,394,315 B2 Aicher Et Al
USOO93943 15B2 (12) United States Patent (10) Patent No.: US 9,394,315 B2 Aicher et al. (45) Date of Patent: Jul.19, 2016 (54) TETRAHYDROI18NAPHTHYRIDINE 6,605,634 B2 8, 2003 Zablocki et al. SULFONAMIDE AND RELATED 6,638,960 B2 10/2003 Assmann et al. 6,683,091 B2 1/2004 Asberomet al. COMPOUNDS FOR USEAS AGONSTS OF 6,828,344 B1 12/2004 Seehra et al. RORY AND THE TREATMENT OF DISEASE 7,084, 176 B2 8, 2006 Morie et al. 7,138.401 B2 11/2006 Kasibhatla et al. (71) Applicant: Lycera Corporation, Ann Arbor, MI 7,329,675 B2 2/2008 Cox et al. 7,420,059 B2 9, 2008 O'Connor et al. (US) 7,482.342 B2 1/2009 D’Orchymont et al. 7,569,571 B2 8/2009 Dong et al. (72) Inventors: Thomas D. Aicher, Ann Arbor, MI (US); 7,696,200 B2 4/2010 Ackermann et al. Peter L. Toogood, Ann Arbor, MI (US); 7,713.996 B2 5/2010 Ackermann et al. Xiao Hu, Northville, MI (US) 7,741,495 B2 6, 2010 Liou et al. 7,799,933 B2 9/2010 Ceccarelli et al. (73) Assignee: Lycera Corporation, Ann Arbor, MI 2006,0004000 A1 1/2006 D'Orchymont et al. 2006/010O230 A1 5, 2006 Bischoff et al. (US) 2007/0010537 A1 1/2007 Hamamura et al. 2007/OO 10670 A1 1/2007 Hirata et al. (*) Notice: Subject to any disclaimer, the term of this 2007/0049556 A1 3/2007 Zhang et al. patent is extended or adjusted under 35 2007/0060567 A1 3/2007 Ackermann et al. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

(19) United States (12) Patent Application Publication (10) Pub
US 20050181041A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0181041 A1 Goldman (43) Pub. Date: Aug. 18, 2005 (54) METHOD OF PREPARATION OF MIXED Related US. Application Data PHASE CO-CRYSTALS WITH ACTIVE AGENTS (60) Provisional application No. 60/528,232, ?led on Dec. 9, 2003. Provisional application No. 60/559,862, ?led (75) Inventor: David Goldman, Portland, CT (US) on Apr. 6, 2004. Correspondence Address: Publication Classi?cation LEYDIG VOIT & MAYER, LTD (51) Int. Cl.7 ....................... .. A61K 31/56; A61K 38/00; TWO PRUDENTIAL PLAZA, SUITE 4900 A61K 9/64 180 NORTH STETSON AVENUE (52) US. Cl. ............................ .. 424/456; 514/179; 514/2; CHICAGO, IL 60601-6780 (US) 514/221 (73) Assignee: MedCrystalForms, LLC, Hunt Valley, (57) ABSTRACT MD This invention pertains to a method of preparing mixed phase co-crystals of active agents With one or more materials (21) Appl. No.: 11/008,034 that alloWs the modi?cation of the active agent to a neW physical/crystal form With unique properties useful for the delivery of the active agent, as Well as compositions com (22) Filed: Dec. 9, 2004 prising the mixed phase co-crystals. Patent Application Publication Aug. 18, 2005 Sheet 1 0f 8 US 2005/0181041 A1 FIG. 1a 214.70°C z.m."m.n... 206.98°C n..0ao 142 OJ/g as:20m=3: -0.8 -1.0 40 90 1:10 2110 Temperture (°C) FIG. 1b 0.01 as:22“.Km: 217 095 24221.4 39Jmum/Q -0.8 35 155 255 255 Temperture (°C) Patent Application Publication Aug. -

High-Throughput H295R Steroidogenesis Assay: Utility As an Alternative and a Statistical Approach to Characterize Effects on Steroidogenesis Derik E
High-throughput H295R steroidogenesis assay: utility as an alternative and a statistical approach to characterize effects on steroidogenesis Derik E. Haggard ORISE Postdoctoral Fellow National Center for Computational Toxicology Computational Toxicology Communities of Practice Dec. 14th, 2017 The views expressed in this presentation are those of the author and do not necessarily reflect the views or policies of the U.S. EPA Outline • Background • Objectives • Assay Background • Methods and Results 1. Evaluation of the HT-H295R assay 2. Development of a quantitative prioritization metric for the HT-H295R assay data • Summary and Conclusions 2 Steroid Hormone Biosynthesis & Metabolism • Proper steroidogenesis is essential: • In utero for fetal development • In adults for reproductive function • Disruption can result in congenital adrenal hyperplasia, sterility, prenatal virilization, salt wasting, etc. • >90% of steroidogenesis occurs in the gonads • Leydig cells (males) or follicular cells (females) • Adrenal gland (corticosteroids) 3 https://www.pharmacorama.com/en/Sections/Androgen_steroid_hormones.php US EPA Endocrine Disruptor Screening Program (EDSP) • EDSP mandated to screen chemicals for endocrine activity (estrogen, androgen, thyroid) • Initial tiered screen relied on low-throughput assays • Modernization of EDSP (EDSP21) to use high-throughput and computational methods • Prioritize the universe of EDSP chemicals for endocrine bioactivity • Altering hormone levels via disruption of biosynthesis or metabolism can also contribute