Appendix ES-7 Hawaiian Tree Snail Propagation Summary
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Linearized Esculentin-2EM Shows Ph Dependent Antibacterial Activity With
Molecular and Cellular Biochemistry https://doi.org/10.1007/s11010-021-04181-7 Linearized esculentin‑2EM shows pH dependent antibacterial activity with an alkaline optimum Erum Malik1 · David A. Phoenix2 · Timothy J. Snape3 · Frederick Harris4 · Jaipaul Singh4 · Leslie H. G. Morton4 · Sarah R. Dennison5 Received: 5 November 2020 / Accepted: 12 May 2021 © The Author(s) 2021 Abstract Here the hypothesis that linearized esculentin 2EM (E2EM-lin) from Glandirana emeljanovi possesses pH dependent activ- ity is investigated. The peptide showed weak activity against Gram-negative bacteria (MLCs ≥ 75.0 μM) but potent efcacy towards Gram-positive bacteria (MLCs ≤ 6.25 μM). E2EM-lin adopted an α-helical structure in the presence of bacterial membranes that increased as pH was increased from 6 to 8 (↑ 15.5–26.9%), whilst similar increases in pH enhanced the ability of the peptide to penetrate (↑ 2.3–5.1 mN m−1) and lyse (↑ 15.1–32.5%) these membranes. Theoretical analysis predicted that this membranolytic mechanism involved a tilted segment, that increased along the α-helical long axis of E2EM-lin (1–23) in the N → C direction, with − < µH > increasing overall from circa − 0.8 to − 0.3. In combination, these data showed that E2EM-lin killed bacteria via novel mechanisms that were enhanced by alkaline conditions and involved the formation of tilted and membranolytic, α-helical structure. The preference of E2EM-lin for Gram-positive bacteria over Gram-negative organisms was primarily driven by the superior ability of phosphatidylglycerol to induce α-helical structure in the peptide as compared to phosphatidylethanolamine. These data were used to generate a novel pore-forming model for the membranolytic activity of E2EM-lin, which would appear to be the frst, major reported instance of pH dependent AMPs with alkaline optima using tilted structure to drive a pore-forming process. -

Testis Development and Differentiation in Amphibians
G C A T T A C G G C A T genes Review Testis Development and Differentiation in Amphibians Álvaro S. Roco , Adrián Ruiz-García and Mónica Bullejos * Departamento de Biología Experimental, Facultad de Ciencias Experimentales, Campus Las Lagunillas S/N, Universidad de Jaén, 23071 Jaén, Spain; [email protected] (Á.S.R.); [email protected] (A.R.-G.) * Correspondence: [email protected]; Tel.: +34-953-212770 Abstract: Sex is determined genetically in amphibians; however, little is known about the sex chromosomes, testis-determining genes, and the genes involved in testis differentiation in this class. Certain inherent characteristics of the species of this group, like the homomorphic sex chromosomes, the high diversity of the sex-determining mechanisms, or the existence of polyploids, may hinder the design of experiments when studying how the gonads can differentiate. Even so, other features, like their external development or the possibility of inducing sex reversal by external treatments, can be helpful. This review summarizes the current knowledge on amphibian sex determination, gonadal development, and testis differentiation. The analysis of this information, compared with the information available for other vertebrate groups, allows us to identify the evolutionarily conserved and divergent pathways involved in testis differentiation. Overall, the data confirm the previous observations in other vertebrates—the morphology of the adult testis is similar across different groups; however, the male-determining signal and the genetic networks involved in testis differentiation are not evolutionarily conserved. Keywords: amphibian; sex determination; gonadal differentiation; testis; sex reversal Citation: Roco, Á.S.; Ruiz-García, A.; Bullejos, M. Testis Development and Differentiation in Amphibians. -

©Copyright 2008 Joseph A. Ross the Evolution of Sex-Chromosome Systems in Stickleback Fishes
©Copyright 2008 Joseph A. Ross The Evolution of Sex-Chromosome Systems in Stickleback Fishes Joseph A. Ross A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2008 Program Authorized to Offer Degree: Molecular and Cellular Biology University of Washington Graduate School This is to certify that I have examined this copy of a doctoral dissertation by Joseph A. Ross and have found that it is complete and satisfactory in all respects, and that any and all revisions required by the final examining committee have been made. Chair of the Supervisory Committee: Catherine L. Peichel Reading Committee: Catherine L. Peichel Steven Henikoff Barbara J. Trask Date: In presenting this dissertation in partial fulfillment of the requirements for the doctoral degree at the University of Washington, I agree that the Library shall make its copies freely available for inspection. I further agree that extensive copying of the dissertation is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Requests for copying or reproduction of this dissertation may be referred to ProQuest Information and Learning, 300 North Zeeb Road, Ann Arbor, MI 48106-1346, 1-800-521-0600, to whom the author has granted “the right to reproduce and sell (a) copies of the manuscript in microform and/or (b) printed copies of the manuscript made from microform.” Signature Date University of Washington Abstract The Evolution of Sex-Chromosome Systems in Stickleback Fishes Joseph A. Ross Chair of the Supervisory Committee: Affiliate Assistant Professor Catherine L. -

Standard Common and Current Scientific Names for North American Amphibians, Turtles, Reptiles & Crocodilians
STANDARD COMMON AND CURRENT SCIENTIFIC NAMES FOR NORTH AMERICAN AMPHIBIANS, TURTLES, REPTILES & CROCODILIANS Sixth Edition Joseph T. Collins TraVis W. TAGGart The Center for North American Herpetology THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY www.cnah.org Joseph T. Collins, Director The Center for North American Herpetology 1502 Medinah Circle Lawrence, Kansas 66047 (785) 393-4757 Single copies of this publication are available gratis from The Center for North American Herpetology, 1502 Medinah Circle, Lawrence, Kansas 66047 USA; within the United States and Canada, please send a self-addressed 7x10-inch manila envelope with sufficient U.S. first class postage affixed for four ounces. Individuals outside the United States and Canada should contact CNAH via email before requesting a copy. A list of previous editions of this title is printed on the inside back cover. THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY BO A RD OF DIRE ct ORS Joseph T. Collins Suzanne L. Collins Kansas Biological Survey The Center for The University of Kansas North American Herpetology 2021 Constant Avenue 1502 Medinah Circle Lawrence, Kansas 66047 Lawrence, Kansas 66047 Kelly J. Irwin James L. Knight Arkansas Game & Fish South Carolina Commission State Museum 915 East Sevier Street P. O. Box 100107 Benton, Arkansas 72015 Columbia, South Carolina 29202 Walter E. Meshaka, Jr. Robert Powell Section of Zoology Department of Biology State Museum of Pennsylvania Avila University 300 North Street 11901 Wornall Road Harrisburg, Pennsylvania 17120 Kansas City, Missouri 64145 Travis W. Taggart Sternberg Museum of Natural History Fort Hays State University 3000 Sternberg Drive Hays, Kansas 67601 Front cover images of an Eastern Collared Lizard (Crotaphytus collaris) and Cajun Chorus Frog (Pseudacris fouquettei) by Suzanne L. -
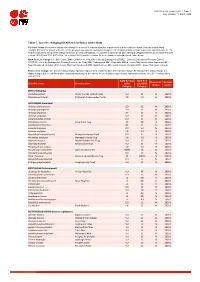
Table 7: Species Changing IUCN Red List Status (2018-2020)
IUCN Red List version 2020-1: Table 7 Last Updated: 19 March 2020 Table 7: Species changing IUCN Red List Status (2018-2020) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2019 (IUCN Red List version 2019-3) and 2020 (IUCN Red List version 2020-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2019) List (2020) change version Category -

Sex Reversal Induced by Steroid Hormones in Glandirana Rugosa Frogs
Central JSM Sexual Medicine Mini Review *Corresponding author Masahisa Nakamura, Waseda Research Institute for Science and Engineering, 3-4-1 Okubo, Shinjuku-ku, Waseda University, Tokyo, 169-8555, Japan, Email: Sex Reversal Induced by Steroid [email protected] Submitted: 01 September 2020 Hormones in Glandirana rugosa Accepted: 15 September 2020 Published: 17 September 2020 ISSN: 2578-3718 Frogs Copyright © 2020 Nakamura M, et al. 1,2 2 2 Masahisa Nakamura *, Akira Oike , and Etsuro Ito OPEN ACCESS 1Waseda Research Institute for Science and Engineering, Waseda University, Japan 2Department of Biology, Waseda University, Japan Abstract In general, sex is determined at fertilization of zygotes by sex chromosome composition; this is known as genotypic sex determination in many vertebrate species. Interestingly, steroid hormones can reverse sex of many species in fish, amphibians and reptiles; androgens induce the female-to-male sex reversal, whereas estrogens cause the male-to-female one. For such sex reversal, a functioning sex-determining gene on the sex chromosome is not required. However, little is known about the mechanisms involved in the sex-reversal at histological and molecular levels. To clarify the mechanism of sex reversal, it is very important to detect the first signs of histological changes in the sex-reversing gonads. For this purpose, we have determined a threshold dosage of steroid hormones to induce sex reversal. When tadpoles of Glandirana (G.) rugosa are reared in water containing a threshold dosage of steroid hormones, genetic females and males form a mixture of testis and ovary, the so-called ovotestis during the transit period of sex reversal. -

Predatory Ecology of the Invasive Wrinkled Frog (Glandirana Rugosa) in Hawai´I
Gut check: predatory ecology of the invasive wrinkled frog (Glandirana rugosa) in Hawai´i By Melissa J. Van Kleeck and Brenden S. Holland* Abstract Invertebrates constitute the most diverse Pacific island animal lineages, and have correspondingly suffered the most significant extinction rates. Losses of native invertebrate lineages have been driven largely by ecosystem changes brought about by loss of habitat and direct predation by introduced species. Although Hawaii notably lacks native terrestrial reptiles and amphibians, both intentional and unintentional anthropogenic releases of herpetofauna have resulted in the establishment of more than two dozen species of frogs, toads, turtles, lizards, and a snake. Despite well-known presence of nonnative predatory species in Hawaii, ecological impacts remain unstudied for a majority of these species. In this study, we evaluated the diet of the Japanese wrinkled frog, Glandirana rugosa, an intentional biocontrol release in the Hawaiian Islands in the late 19th century. We collected live frogs on Oahu and used museum collections from both Oahu and Maui to determine exploited diet composition. These data were then compared to a published dietary analysis from the native range in Japan. We compiled and summarized field and museum distribution data from Oahu, Maui, and Kauai to document the current range of this species. Gut content analyses suggest that diet composition in the Hawaiian Islands is significantly different from that that in its native Japan. In the native range, the dominant taxonomic groups by volume were Coleoptera (beetles), Lepidoptera (moths, butterflies) and Formicidae (ants). Invasive frogs in Hawaii exploited mostly Dermaptera (earwigs), Amphipoda (landhoppers) and Hemiptera (true bugs). -

Larval Systematics of the Peninsular Malaysian Ranidae (Amphibia: Anura)
LARVAL SYSTEMATICS OF THE PENINSULAR MALAYSIAN RANIDAE (AMPHIBIA: ANURA) LEONG TZI MING NATIONAL UNIVERSITY OF SINGAPORE 2005 LARVAL SYSTEMATICS OF THE PENINSULAR MALAYSIAN RANIDAE (AMPHIBIA: ANURA) LEONG TZI MING B.Sc. (Hons.) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF BIOLOGICAL SCIENCES THE NATIONAL UNIVERSITY OF SINGAPORE 2005 This is dedicated to my dad, mum and brothers. i ACKNOWLEDGEMENTS I am grateful to the many individuals and teams from various institutions who have contributed to the completion of this thesis in various avenues, of which encouragement was the most appreciated. They are, not in any order of preference, from the National University of Singapore (NUS): A/P Peter Ng, Tan Heok Hui, Kelvin K. P. Lim, Darren C. J. Yeo, Tan Swee Hee, Daisy Wowor, Lim Cheng Puay, Malcolm Soh, Greasi Simon, C. M. Yang, H. K. Lua, Wang Luan Keng, C. F. Lim, Yong Ann Nee; from the National Parks Board (Singapore): Lena Chan, Sharon Chan; from the Nature Society (Singapore): Subaraj Rajathurai, Andrew Tay, Vilma D’Rozario, Celine Low, David Teo, Rachel Teo, Sutari Supari, Leong Kwok Peng, Nick Baker, Tony O’Dempsey, Linda Chan; from the Wildlife Department (Malaysia): Lim Boo Liat, Sahir bin Othman; from the Forest Research Institute of Malaysia (FRIM): Norsham Yaakob, Terry Ong, Gary Lim; from WWF (Malaysia): Jeet Sukumaran; from the Economic Planning Unit, Malaysia (EPU): Puan Munirah; from the University of Sarawak (UNIMAS): Indraneil Das; from the National Science Museum, Thailand: Jairujin Nabhitabhata, Tanya Chan-ard, Yodchaiy Chuaynkern; from the University of Kyoto: Masafumi Matsui; from the University of the Ryukyus: Hidetoshi Ota; from my Indonesian friends: Frank Bambang Yuwono, Ibu Mumpuni (MZB), Djoko Iskandar (ITB); from the Philippine National Museum (PNM): Arvin C. -

Catch and Culture Aquaculture - Environment
Aquaculture Catch and Culture Aquaculture - Environment Fisheries and Environment Research and Development in the Mekong Region Volume 27, No 2 ISSN 0859-290X August 2021 INSIDE Mitsubishi joins Lao wind project to export power to Viet Nam Water quality still ‘good’ or ‘excellent’ at most sites across Lower Mekong Mud-free farming of eels gathers pace in Mekong Delta Study stresses need to identify organisms at greatest risk from plastics Climate risks moderately to highly negative for Mekong credit ratings How ASEAN central banks are managing climate and environment risks August 2021 Catch and Culture - Environment Volume 27, No. 2 1 Aquaculture Catch and Culture - Environment is published three times a year by the office of the Mekong River Commission Secretariat in Vientiane, Lao PDR, and distributed to over 650 subscribers around the world. The preparation of the newsletter is facilitated by the Environmental Management Division of the MRC Secretariat. Free email subscriptions are available through the MRC website, www.mrcmekong.org. For information on the cost of hard-copy subscriptions, contact the MRC’s Documentation Centre at [email protected]. Contributions to Catch and Culture - Environment should be sent to [email protected] and copied to [email protected]. Editorial Panel: Hak Socheat, Director of Environmental Management Division So Nam, Chief Environmental Management Officer Prayooth Yaowakhan, Ecosystem and Wetland Specialist Nuon Vanna, Fisheries and Aquatic Ecology Officer Ly Kongmeng, Water Quality Officer Erinda Pubill Panen, Environmental Monitoring Advisor, GIZ-MRC Cooperation Programme Mayvong Sayatham, Environmental Diplomacy Advisor, GIZ-MRC Cooperation Programme Editor: Peter Starr Designer: Chhut Chheana Associate editor: Michele McLellan The MRC is funded by contributions from its Member Countries and development partners of Australia, Belgium, the European Union, Finland, France, Germany, Japan, Luxembourg, the Netherlands, Sweden, Switzerland, the United States of America and the World Bank. -

Amphibian Population Declines and Chytridiomycosis in South Korea by Mi-Sook Min, Hang Lee, & Bruce Waldman
Regional Insight Amphibian Population Declines and Chytridiomycosis in South Korea By Mi-Sook Min, Hang Lee, & Bruce Waldman orea has a diverse, but understudied, amphibian fauna the spread of the amphibian chytrid fungus, Batrachochytrium comprising 18 species of which only two are considered dendrobatidis (Bd). Kto be of concern on the IUCN Red List. The systematics of the four species of hynobiid salamanders species have been Prevalence of amphibian chytrid fungus in South Korea well studied (Baek et al. 2011), but little is known about their We are studying how the amphibian chytrid fungus affects Korean ecology. Two endemic species, the Jeju salamander, Hynobius species and its possible contribution to population declines. quelpaertensis, confined to Jeju Island and southern regions, Although no mass mortality events have been reported, nor have and the Kori salamander, H. yangi , found in the southeast of the any individuals from the wild been observed demonstrating country, resemble the more widely distributed Korean salamander clinical signs of chytridiomycosis among the Korean amphibians, H. leechii and previously were classified as subspecies. Further population sizes have declined and ranges have contracted in work may reveal at least several species. Habitat three additional species degradation and destruction in this group. A lungless may be primary causes, as salamander, Karsenia well as harvesting for food koreana, was discovered or medicine especially in recently and represents an rural areas, but disease enigma as the only known also may play a role. Since plethodontid in Asia, but it is 2007, we have surveyed not genetically close to North amphibians throughout the American Plethodon (Min et Korean peninsula and Jeju al. -

July to December 2019 (Pdf)
2019 Journal Publications July Adelizzi, R. Portmann, J. van Meter, R. (2019). Effect of Individual and Combined Treatments of Pesticide, Fertilizer, and Salt on Growth and Corticosterone Levels of Larval Southern Leopard Frogs (Lithobates sphenocephala). Archives of Environmental Contamination and Toxicology, 77(1), pp.29-39. https://www.ncbi.nlm.nih.gov/pubmed/31020372 Albecker, M. A. McCoy, M. W. (2019). Local adaptation for enhanced salt tolerance reduces non‐ adaptive plasticity caused by osmotic stress. Evolution, Early View. https://onlinelibrary.wiley.com/doi/abs/10.1111/evo.13798 Alvarez, M. D. V. Fernandez, C. Cove, M. V. (2019). Assessing the role of habitat and species interactions in the population decline and detection bias of Neotropical leaf litter frogs in and around La Selva Biological Station, Costa Rica. Neotropical Biology and Conservation 14(2), pp.143– 156, e37526. https://neotropical.pensoft.net/article/37526/list/11/ Amat, F. Rivera, X. Romano, A. Sotgiu, G. (2019). Sexual dimorphism in the endemic Sardinian cave salamander (Atylodes genei). Folia Zoologica, 68(2), p.61-65. https://bioone.org/journals/Folia-Zoologica/volume-68/issue-2/fozo.047.2019/Sexual-dimorphism- in-the-endemic-Sardinian-cave-salamander-Atylodes-genei/10.25225/fozo.047.2019.short Amézquita, A, Suárez, G. Palacios-Rodríguez, P. Beltrán, I. Rodríguez, C. Barrientos, L. S. Daza, J. M. Mazariegos, L. (2019). A new species of Pristimantis (Anura: Craugastoridae) from the cloud forests of Colombian western Andes. Zootaxa, 4648(3). https://www.biotaxa.org/Zootaxa/article/view/zootaxa.4648.3.8 Arrivillaga, C. Oakley, J. Ebiner, S. (2019). Predation of Scinax ruber (Anura: Hylidae) tadpoles by a fishing spider of the genus Thaumisia (Araneae: Pisauridae) in south-east Peru. -

Distribution and Genetic Diversity of the Amphibian Chytrid in Japan
Journal of Fungi Article Distribution and Genetic Diversity of the Amphibian Chytrid in Japan Koichi Goka 1,*, Jun Yokoyama 2 and Atsushi Tominaga 3 1 National Institute for Environmental Studies, 16-2 Onogawa, Tsukuba 305-8506, Japan 2 Department of Biology, Faculty of Sciences, Yamagata University, 1-4-12 Kojirakawa, Yamagata-shi, Yamagata 990-8560, Japan; [email protected] 3 Department of Natural Sciences, Faculty of Education, University of the Ryukyus, 1 Senbaru, Nishihara, Okinawa 901-0213, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-29-850-2480; Fax: +81-29-850-2582 Abstract: While research on frog chytrid fungus Batrachochytrium dendrobatidis (Bd), an infectious disease that threatens amphibian diversity, continues to advance worldwide, little progress has been made in Japan since around 2010. The reason for this is, which we pointed out in 2009, that the origin of frog chytrid fungus may be in the East Asian region, including Japan based on the Bd ITS-DNA variation, and as few cases of mass mortality caused by this fungus have been observed in wild amphibian populations in Japan, the interest of the Japanese government and the general public in Bd has waned. However, we believe that organizing the data obtained so far in Japan and distributing the status of frog chytrid fungus in Japan to the world will provide useful insight for future risk management of this pathogen. We collected more than 5500 swab samples from wild amphibians throughout Japan from 2009 to 2010. Then, we investigated the infection status using the Nested-PCR method.