Pesticide Residues in Vegetables Collected from Different Markets of Navsari District of India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 Anjuman Final Ahewal
786/92 :YF5GF o 1997 ;BFJT o OZDFG GAJL C{ ;BFJT ACL:T SF V[S NZbT C[4 _;SL XFB[ HDLG 5Z h]SL C]. C{4 _; G[ >; SL SL;L XFB SF[ YFD ,LIF4 JF[ >;[ HgGT D[\ ,[ HFI[\U[[P V\H]DG[ lZOF> R[ZL8[A, 8=:8 AL,LDF[ZFP Z_:80” 8=:8 G\AZ o JSOí))!í!((( GJ;FZL UF{Q[ VFhD V[HI]S[XG, V[g0 NLGL TF,LD tYF bJFhF UZLA GJFh D[0LS, ZL,LO O\0 JFlQ”S VC[JF, VG[ lZ5F[8” JQ” o Z)Z) < Z)Z! Our Website : www.Anjuman-e-Refai.org. <o 5|l;wW STF” o< CF_ VaN],CDLN _P D]ÿ,F\ CF_ ;],[DFGEF> V[;P 58[, CF_ VÿTFOC]X[G >A|FCLD Z[\8LIF CF_ DF[C\DN >SAF, V[P SF[,LIF DF[>GAFAF D]:TFS RZLJF,F ANJUMAN-E- REFAI CHERITABLE TRUST-BILIMORA Trustee Board No. Name Address Photo 1 Haji Abdul Hamid Haji GulamMohammed Mulla Station Road, Near by Station Masjid, Trustee Bilimora - 396 321 PhonePhone : 285444 Mo. : 9904278692 2 Haji Suleman Saleh Patel Sanket Appartment, Trustee M. G. Road, Bilimora - 396 321 Phone : 286344 Mo. : 9426889300 3 Haji Mohammed Iqbal Jawahar Road, Haji Ahmedbhai Koliya Again Post Office Trustee Bilimora - 396 321 Phone : 279786 Mo. : 9925555780 4 Haji AltafHusain Ibrahim 1072, Bangia Faliya, Rentia Bilimora - 396 321 Trustee Phone : 286137 Mo. : 9825119213 5 Moinbaba Mustak Chariwala 1072, Bangia Faliya, Trustee Bilimora - 396 321 Mo. : 9725586863 &*^Í(Z 8=:8GL :YF5GF !((& V\H]DG[ lZOF> R[ZL8[A, 8=:84 AL,LDF[ZF D[G[_\U 8=:8LGL S,D[YL V:;,FDF[ V,IS]D4 JPJP VÿCdN]l,ÿ,FCL ZaAL, VF,DLG J:;,FT] J:;,FD] V,F ;liINL, D]Z;,LGP ;J[” TFZLOG[ 5FShFT DF8[ K[4 H[6[ ;DU| ;’lq8G]\ ;H”G SI©] VG[ ,FBF[ SZF[0F[ N]~NF[< ;,FD < ;ZSFZ[<NF[<VF,D ;ÿ,FCF[ V,IC[ J:;,D 5Z H[DG[ Vÿ,FC TVF,FV[ -

District Human Development Report of Navsari
For Office Use Only District Human Development Report Gujarat Social Infrastructure Development Society (GSIDS) General Administration Department (Planning) Government of Gujarat Sector-18, Gandhinagar District Human Development Report NAVSARI Gujarat Social Infrastructure Development Society (GSIDS) General Administration Department (Planning) Government of Gujarat DISTRICT HUMAN DEVELOPMENT REPORT: NAVSARI Copyright : 2016 Gujarat Social Infrastructure Development Society (GSIDS), GoG Published : Gujarat Social Infrastructure Development Society (GSIDS), Government of Gujarat By First : 2016 Published All rights reserved. No part of this publication may be reproduced, stored or transmitted in any form by any means without the prior permission from the publisher. This Report does not necessarily reflect the views of the Gujarat Social Infrastructure Development Society (GSIDS). While every care has been taken to reproduce the accurate data, oversights / errors may occur. If found convey it to the Gujarat Social Infrastructure Development Society (GSIDS). Designed by Tejal Parmar, SPAC, GSIDS The report is prepared by Shri Jairambhai Patel Institute of Business Management, Gandhinagar under a tripartite MoU between Member Secretary, the Gujarat Social Infrastructure Development Society (GSIDS), District Collector, Porbandar and Shri Jairambhai Patel Institute of Business Management (formerly known as NICM) (Post Graduate Centre of Gujarat Technological University), Gandhinagar. Gujarat Social Infrastructure Development Society (GSIDS) General Administration Department (Planning) Government of Gujarat MESSAGE Human Development is a development paradigm which is beyond mere rise or fall of national incomes. It is about creating an environment where people can develop their full potential and lead productive, creative lives in accordance with their needs and interests. People are the real wealth of nation. Development is thus about expanding the choices people have to lead lives that they value. -
7. Additional Studies
Dahej Nagothane Ethane Pipeline (DNEPL) Project Chapter-7 Additional Studies 7. ADDITIONAL STUDIES 7.1. Quantitative Risk Assessment Risk analysis study was conducted for DNEPL originating from RIL DMD in Gujarat to RIL NMD in Maharashtra with a spur pipeline to RIL HMD. Conducting a Quantitative Risk Assessment (QRA) is important in the management of risk, as cross country pipelines transport hazardous material traversing several hundreds of kilometres through villages, forests, rivers, roads, rail crossings, etc. Risk Assessment study was undertaken to address the hazards and risks associated with pipeline transportation of hydrocarbons as per requirement of MoEFCC. The goal of risk assessment is to estimate the risk to personal, property and public along the pipeline route from the unlikely event of leaks and/or rupture during pipeline operation. In addition, results of such studies provide help in improvement of safety by recommending measures to risk to the extent possible. This section outlines the Quantitative Risk Assessment (QRA) Report prepared by M/s Bureau Veritas. 7.1.1. Project Details The proposed pipeline will transfer liquid Ethane made available from RIL DMD to RIL NMD and HMD through 12” dia mainline and 8” dia spur pipeline respectively. One pump station is proposed to be located within RIL DMD. The pipeline will be constructed as per OISD 214 and ASME B 31.4 standards. Table 100: Salient details of the proposed pipeline Dahej Nagothane Ethane Pipeline Project–Salient Details Description UoM Details Design Throughput -

Brief Industrial Profile of NAVSARI District
Government of India Ministry of MSME Brief Industrial Profile of NAVSARI District MSME – DEVELOPMENT INSTITUTE Govt. of India Ministry of Micro, Small & Medium Enterprises Harsiddh Chambers, 4th Floor, Ashram Road, Ahmedabad-380 014 Tel.No. 079-27543147 & 27544248, Fax No.079-27540619 E-mail : [email protected] Website: www.msmediahmedabad.gov.in CONTENTS -1- Brief Industrial Profile of Navsari District 1. General Characteristics of the District: 1.1 Location & Geographical Area : Navsari district is located between 20.07’- 21.00’ North Latitude and 72.43’-73.00’ East Longtitude.The district is located in the South Eastern part of Gujarat state in the coastal low land along Purna river. The total geographical areas of Navsari district is 2657.56 Sq.Kms. It is a coastal district open to the Arabian Sea from West and is bounded by Valsad disrict on the North. On the East, it is contiguous to Dand district in Gujarat. 1.1.2 Topography : Navsari district was craved out on the erstwhile – unified Valsad district with effect from 2nd October, 1997. The district can be distinctly divided into three Agro-climatically divisons 1) Forest and hilly tracts of the Eastern parts comprising Vansda bloct. 2) Saline Soils due to inundation by sea of Western parts comprising parts in Gandevi and Jalalpore blocks. 3) Black Fertile soils of the central parts comprising Navsari, Gandevi and Chikhl Blocks. The area between East and West of Navsari District is known as “Green Belt” which is suitable for gardening and nuserries. # Navsari district offers good scope for entrepreneurs to establish new industrial units for manufacturing various products. -

Trade Marks Journal No: 1904 , 03/06/2019 Class 11
Trade Marks Journal No: 1904 , 03/06/2019 Class 11 2332396 16/05/2012 ESHDEEP SINGH BHASIN 227 AMBA TOWERS DC CHOWK SEC-9 ROHINI DELHI-85 MANUFACTURER & MERCHANTS Address for service in India/Attorney address: M ABHIJNAN R-104, (BASEMENT) GREATER KAILASH, PART-1, NEW DELHI-110048 Used Since :01/01/2009 DELHI CFL AND LED LIGHTS REGISTRATION OF THIS TRADE MARK SHALL GIVE NO RIGHT TO THE EXCLUSIVE USE OF THE.WORDS i.e., LUMEN BRIGHT TECHNOLOGY and MARK TO BE USED AS A WHOLE. 2219 Trade Marks Journal No: 1904 , 03/06/2019 Class 11 2547253 12/06/2013 NEELAM KAPOOR SUNIL KAPOOR trading as ;KAPOOR AGENCIES 2246/2247 H C SAIN MARG CHANDNI CHOWK NEAR JUBLEE CINEMA DELHI 6 MANUFACTURERS AND MERCHANTS Address for service in India/Attorney address: CONCEPTLEGAL 159-E KAMLA ANGAR NEW DELHI 110007 Used Since :10/12/2012 DELHI ELECTRIC FANS, COOLER, MIST FANS, AIR COOLING SYSTEM 2220 Trade Marks Journal No: 1904 , 03/06/2019 Class 11 2555797 27/06/2013 SHOP CJ NETWORK PRIVATE LIMITED. 6TH FLOOR, STAR CJ PLAZA, DR. D.B. MARG, GRANT ROAD (EAST), MUMBAI-400007 MANUFACTURER, TRADER AND MERCHANT A PRIVATE LIMITED COMPANY Address for service in India/Agents address: DUA ASSOCIATES. 202 - 206, TOLSTOY HOUSE 15, TOLSTOY MARG, NEW DELHI - 110 001. Proposed to be Used To be associated with: 2546916 MUMBAI VACUUM CLEANER, , AIR COOLER THE MARK SHALL BE LIMITED TO THE COLOURS AS SHOWN IN THE REPRESENTATION ON THE FORM OF THE APPLICATION. THIS IS CONDITION OF REGISTRATION THAT BOTH/ALL LABELS SHALL BE USED TOGETHER. -

Form I Miniing Activity Project (Blacktrap) Manubhai
FORM I FOR MINIING ACTIVITY PROJECT (BLACKTRAP) EXISTING MINOR MINERAL BLACKTRAP OTHER THEN SAND MINING LEASE PROJECT AT MANUBHAI SONKABHAI MAHALA SURVEY NO: BLOCK NO - 262 - 4.80.00 HA AT:-JAMANPADA TA:-KHERGAM DI:-NAVSARI STATE:-GUJARAT MO.NO:-9825296279 1 MANUBHAI SONKABHAI MAHALA AT-JAMANPADA, TA-KHERGAM, DIST-NAVSARI Co. District Level Expert Appraisal Committee, ………………………………………………….., ………………………………………………….., ……………………………….., Gujarat. Kind Attn:-MANUBHAI SONKABHAI MAHALA Subject:- Submission Of Form 1, and Soft Copy for EC Application For Environmental Clearance For “BLACKTRAP” EXISTING MINOR MINERAL BLACKTRAP OTHER THEN SAND MINING LEASE PROJECT Mining Project (Area – 4.80.00 haq) located At:-JAMANPADA Ta:- KHERGAM Di:-NAVSARI Dear Sir, Please find enclosed application form (Form-1) along with enclosures for getting the environmental clearance for our “BLACKTRAP” EXISTING MINOR MINERAL BLACKTRAP OTHER THEN SAND MINING LEASE PROJECT Mining Projecct (Area - 4.80.00 ha) At:-JAMANPADA Ta:- KHERGAM Di:-NAVSARI(Gujarat). As per the EIA Notification 2006, environment Clearance is required for mining of minerals under category 1(a) for area of 5 Ha and more. The MOEF vide OM dated -18th May in reference to hon’ble Supreme Court order in the matter of Deepakkumar etc Vs State of Haryana & Others, clarified that mining projects with lease area up to less than 50 Ha including of prrojects of mineral mines with lease area less than 5 Ha. Would be treated as category ‘B’ As defined in EIA Notification 2006 and will be considered by the respective EIAA. We request you to process our application and accord us the environmental clearance at the earliest as per the Hon’ble supreme court order dated:-27/02/2012 2 “A” Request Letter To, Respected, Member Secretary, S.E.A.C., Paryavaran Bhavan, Sec.,10/A, Gandhinagar, Dear sir, With respect, I above said President MANUBHAI SONKABHAI MAHALA got EXISTING MINOR MINERAL BLACKTRAP OTHER THEN SAND MINING LEASE PROJECT of minor mineral “BLACKTRAP” from Collector NAVSARI on At:-JAMANPADA Ta:- KHERGAM Di:-NAVSARI(Gujarat). -

Zakat - Lillah - Sadaqah Helping Families Living in Deprived Areas to Have a Better Quality of Life
Zakat - Lillah - Sadaqah Helping families living in deprived areas to have a better quality of life. Registered Charity No:1067746 The Projects With the generosity seen through the donations we have been able to help rebuild dilapidated homes and give a number of people equipment and assistance to start their own income source. Some of these include: Rebuild of 5 houses All the houses were falling apart with an entire family, including daughter in-laws, sharing one room which was their day room and bedroom. This left little priva- cy for the household. The homes were rebuilt, separating rooms and intro- ducing basic essentials like a simple kitchen area and toilets. A water hand pump was also installed on a location serving four of the houses. Empowerment Projects – Several vendors have been set up with transport and merchandise to begin bringing in an income. This includes selling vegetables, chickens, spices etc with each vendor being given a different set of goods to sell. - Hand carts (lary) were purchased and given to some existing vendors who were struggling with broken equipment. - A bicycle repair shop was helped with the purchase of mainly tyres to help boost his business. - A woman was helped through the purchase of a sew- ing machine through which she now earns as a ladies tailor. - Help was given to set an individual up with a mobile phone repair shop. - An individual was helped through purchase to start selling hosiery - Around four individuals in different regions were provided with plumbing tools. - An individual has been helped through the purchase of a fishing net. -

List of Beneficiaries Navsari Camp
LIST OF BENEFICIARIES NAVSARI CAMP S.No Name Father name Address Phone no Age M/F catego Disability Category ry 1 2016-17/00405 2016-17/NAVS2/T17/00400 AAMIR ABDUL BHAI AMBHER,JALALPORE 8128765108 12 M OBC MENTALLY IMPAIRED(40 %) 2 2016-17/04619 2016-17/NAV47/T8/02124 JUBER MOHD. MOHD. KADU NASILPORE,NAVSARI 9909097509 17 M General MENTALLY IMPAIRED(40 %) 3 2016-17/04618 2016-17/NAV47/T8/02123 FAIJAN AMIN MIYA NANA PANDHA,NAVSARI 9825856128 18 M SC MENTALLY IMPAIRED(40 %) 4 2016-17/04604 2016-17/NAV47/T8/02109 SHAILESH BHAI DINESH BHAI RAM NAGAR,NAVSARI 8141814494 17 M ST MENTALLY IMPAIRED(40 %) 5 2016-17/04601 2016-17/NAV47/T8/02106 MAYUR DHIRU BHAI VAPI,NAVSARI 9925949004 13 M SC MENTALLY IMPAIRED(40 %) 6 2016-17/04590 2016-17/NAV47/T8/01993 AFRIN ASHIF MAGDUMPURA,NAVSARI 9687745627 13 F ST MENTALLY IMPAIRED(75 %) 7 2016-17/04588 2016-17/NAV47/T8/01991 PAYAL UMESH NANI CHOISI,NAVSARI 9727364785 10 F OBC MENTALLY IMPAIRED(50 %) 8 2016-17/04585 2016-17/NAV47/T8/01988 LAVYA PANDAY KUMAR LAV 641 KRUSHNA NAGAR,NAVSARI 9727704004 8 M General MENTALLY IMPAIRED(50 %) 9 2016-17/04568 2016-17/NAV47/T8/01971 GAMIT BHAI RITESH BHAI MAHUVAS,NAVSARI 9913290377 12 M General MENTALLY IMPAIRED(75 %) 10 2016-17/04563 2016-17/NAVS46/T8/01903 DASHRATH BALU BHAI SATADIYA,CHIKHLI 7567874404 15 M ST MENTALLY IMPAIRED(75 %) 11 2016-17/04559 2016-17/NAVS46/T8/01899 DIPEN HARESH BHAI NADI FALIYA,CHIKHLI 14 M ST MENTALLY IMPAIRED(50 %) 12 2016-17/04541 2016-17/NAVS46/T8/01881 BHAVNA BEN DEEPAK PANI KHADAK,CHIKHLI 9904654872 23 F General MENTALLY IMPAIRED(40 %) -
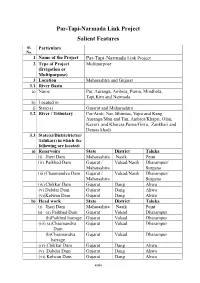
Par-Tapi-Narmada Link Project Salient Features Sl
Par-Tapi-Narmada Link Project Salient Features Sl. Particulars No. 1 Name of the Project Par-Tapi-Narmada Link Project 2 Type of Project Multipurpose (Irrigation or Multipurpose) 3 Location Maharashtra and Gujarat 3.1 River Basin a) Name Par, Auranga, Ambica, Purna, Mindhola, Tapi,Kim and Narmada b) Located in i) State(s) Gujarat and Maharashtra 3.2 River / Tributary Par/Aroti, Nar, Bhimtas, Vajra and Keng Auranga/Man and Tan, Ambica/Khapri, Olan, Kaveri and Kharera,Purna/Girra, Zankhari and Damas khadi 3.3 State(s)/Districtrict(s)/ Taluka(s) in which the following are located: a) Reservoirs State District Taluka (i) Jheri Dam Maharashtra Nasik Peint (ii) Paikhed Dam Gujarat / Valsad/Nasik Dharampur/ Maharashtra Surgana (iii)Chasmandva Dam Gujarat / Valsad/Nasik Dharampur/ Maharashtra Surgana (iv) Chikkar Dam Gujarat Dang Ahwa (v) Dabdar Dam Gujarat Dang Ahwa (vi)Kelwan Dam Gujarat Dang Ahwa b) Head work State District Taluka (i) Jheri Dam Maharashtra Nasik Peint (ii) (a) Paikhed Dam Gujarat Valsad Dharampur (b)Paikhed barrage Gujarat Valsad Dharampur (iii) (a)Chasmandva Gujarat Valsad Dharampur Dam (b)Chasmandva Gujarat Valsad Dharampur barrage (iv) Chikkar Dam Gujarat Dang Ahwa (v) Dabdar Dam Gujarat Dang Ahwa (vi) Kelwan Dam Gujarat Dang Ahwa xxxiv c) Command Area State District Taluka 1. Enroute command Gujarat Dang Ahwa area Bharuch Ankleshwar, Valia Jhagadia Navsari Vansda Surat Mangrol,Mahuva, Mandvi Tapi Vyara Valsad Dharampur 2. Projects proposed by Gujarat Navsari Vansda,Chikhali Government of Khergam Gujarat Valsad Kaprada, Valsad, Dharampur Tapi Vyara,Songadh Surat Mahuva 3. Right side command Gujarat Tapi Vyara area by lift Tapi Songadh Surat Mangrol, Umarpada Bharuch Jhagadia, Valia 4. -

Dadabhai Naoroji and the Evolution of the Demand for Indian Self-Government
The Grand Old Man: Dadabhai Naoroji and the Evolution of the Demand for Indian Self-Government The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Patel, Dinyar Phiroze. 2015. The Grand Old Man: Dadabhai Naoroji and the Evolution of the Demand for Indian Self-Government. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:17467241 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA The Grand Old Man: Dadabhai Naoroji and the Evolution of the Demand for Indian Self-Government A dissertation presented by Dinyar Patel to The Department of History In partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of History Harvard University Cambridge, Massachusetts May 2015 © 2015 Dinyar Patel All rights reserved. Dissertation Advisor: Professor Sugata Bose Dinyar Patel The Grand Old Man: Dadabhai Naoroji and the Evolution of the Demand for Indian Self-Government Abstract This dissertation traces the thought and career of Dadabhai Naoroji, arguably the most significant Indian nationalist leader in the pre-Gandhian era. Naoroji (1825-1917) gave the Indian National Congress a tangible political goal in 1906 when he declared its objective to be self-government or swaraj. I identify three distinct phases in the development of his political thought. -
10Th CRM Report 2016
HEALTH AL M N IS S O I I O T N A N Tenth COMMON REVIEW MISSION Report 2016 HEALTH AL M N IS S O I I O Tenth T N A crm N Common Review Mission Report 2016 This report has been synthesised and published on behalf of the National Health Mission by its technical support institution; National Health Systems Resource Centre (NHSRC) located at NIHFW campus, Baba Gangnath Marg, New Delhi-110 067. We gratefully acknowledge the contributions made by consultants and officers in the NHM Division of the MoHFW. We also place on record our deep appreciation and gratitude to participants from other Ministries, Public Health Institutions, Civil Society and Development Partners who have all contributed to this Common Review Mission Report. ©2016 Ministry of Health and Family Welfare Government of India, Nirman Bhawan New Delhi-110 011 Reproduction of any excerpts from this documents does not require permission from the publisher so long it is verbatim, is meant for free distribution and the source is acknowledged. ISBN: 978-93-82655-19-0 Design & Printed by: Macro Graphics Pvt. Ltd. (www.macrographics.com) MESSAGE Common Review Missions (CRMs) have played a key role as a key monitoring tool particularly for assessing the progress of National Health Mission (NHM) implementation on ground. It has proven to be a valuable mechanism for the Ministry of Health & Family Welfare to review programme and policy changes and I am sure CRM reports have proved useful to States as an independent feedback about their strengths and weaknesses in programme implementation. -
Navsari-Msme.Pdf
VG-2015 MSME - Approved Investment Intentions District : Navsari Sr.No. Name of Company Office Address 1 A- One Lube Refinery. Plot No. 458/1 , GIDC Navsari,,Navsari-396445,Navsari,Navsari 2 Aditya Plastic N.H.No.8, At-Padgha, Dholapipla, Navsari,,Padgha-396445,Navsari,Navsari 3 ADITYA TIMPACK PVT LTD BLOCK NO 61P1 VALOTI GANDEVI ROAD BILIMORA 396380,TA GANDEVI DIST NAVSARI,Valoti-396380,Gandevi,Navsari 4 Ajanta Stone Industries Block No.2011, Bamanvel, Alipore, Chikhali,,Bamanvel-396409,Chikhali,Navsari 5 Ashirwad Food Products Plot No. C1B-29/1, GIDC Bilimora,,-,Navsari 6 Aum Rubber Products Shed No. 122 , GIDC Antaliya , Bilimora ,DIst- Navsari,,Antaliya-396321,Gandevi,Navsari 7 Bcare Contaiener K-1 A, Antaliya, G.I.D.C., Gandevi, Navsari,,Antaliya-396325,Gandevi,Navsari 8 Beena Engineering Corporaton Shed No. K1/6 GIDC Antaliya ,Bilimora ,Dist -Navsari,,Antaliya-396321,Gandevi,Navsari 9 Bhavya Tubes Pvt.Ltd. Shed No. J-67/2 ,GIDC Antalia , Bilimora, Dist -Navsari,,Antaliya-396325,Gandevi,Navsari 10 Deep Sleep L-178/2, Opp. Ark Engineering,Antaliya, GIDC, Bilimora,Antaliya-396325,Gandevi,Navsari 11 Deepak Wood Agency plot no.193,GIDC,Bilimora,Antaliya,,Antaliya-396321,Gandevi,Navsari 12 Devguru Capliners Plot No. L-180/2, GIDC Bilimora,,-,Navsari 13 Devi Engineering Plot No.3,4, Sai Complex, Nr. Cebon Apperal Pvt. Ltd., Udhyog Nagar, Navsari,,Navsari-396445,Navsari,Navsari 14 Dhara Food Samroli, Ghod Faliya, Ta.Chikhali, Dist.Navsari,,Samaroli-396521,Chikhali,Navsari 15 Dhara Water Samroli Faliya, Ta.Chikhali, Dist-Navsari,,Samaroli-396521,Chikhali,Navsari 16 Dinesh Plastic Products Block No.338/2, B-Type, B/h.