Understanding the Genetics of Deafness a Guide for Patients and Families
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
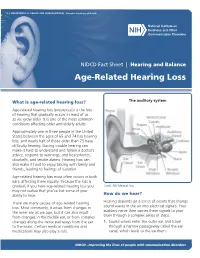
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

Benefits of Exome Sequencing in Children with Suspected Isolated
G C A T T A C G G C A T genes Article Benefits of Exome Sequencing in Children with Suspected Isolated Hearing Loss Roxane Van Heurck 1, Maria Teresa Carminho-Rodrigues 1, Emmanuelle Ranza 1, Caterina Stafuzza 2, Lina Quteineh 1, Corinne Gehrig 1, Eva Hammar 1, Michel Guipponi 1, Marc Abramowicz 1, Pascal Senn 2 , Nils Guinand 2, Helene Cao-Van 2 and Ariane Paoloni-Giacobino 1,* 1 Division of Genetic Medicine, Geneva University Hospitals, 1205 Geneva, Switzerland; [email protected] (R.V.H.); [email protected] (M.T.C.-R.); [email protected] (E.R.); [email protected] (L.Q.); [email protected] (C.G.); [email protected] (E.H.); [email protected] (M.G.); [email protected] (M.A.) 2 Ear-Nose-Throat/Head and Neck Surgery Division, Geneva University Hospitals, 1205 Geneva, Switzerland; [email protected] (C.S.); [email protected] (P.S.); [email protected] (N.G.); [email protected] (H.C.-V.) * Correspondence: [email protected] Abstract: Purpose: Hearing loss is characterized by an extensive genetic heterogeneity and remains a common disorder in children. Molecular diagnosis is of particular benefit in children, and permits the early identification of clinically-unrecognized hearing loss syndromes, which permits effective clinical management and follow-up, including genetic counselling. Methods: We performed whole-exome Citation: Van Heurck, R.; sequencing with the analysis of a panel of 189 genes associated with hearing loss in a prospective Carminho-Rodrigues, M.T.; Ranza, E.; cohort of 61 children and 9 adults presenting mainly with isolated hearing loss. -

Dissertationes Medicinae Universitatis Tartuensis 178
DISSERTATIONES MEDICINAE UNIVERSITATIS TARTUENSIS 178 DISSERTATIONES MEDICINAE UNIVERSITATIS TARTUENSIS 178 RITA TEEK The genetic causes of early onset hearing loss in Estonian children Department of Paediatrics, University of Tartu, Tartu, Estonia Dissertation is accepted for commencement of the degree of Doctor of Medical Sciences on September 22, 2010 by the Council of the Faculty of Medicine, University of Tartu, Estonia. Supervisors: Professor Katrin Õunap, MD, PhD, Department of Paediatrics, University of Tartu, Tartu, Estonia The Late Professor Mart Kull, MD, PhD, Department of Oto-Rhino-Laryngology, University of Tartu, Tartu, Estonia (2005–2008) Reviewers: Assistant Professor Gunnar Tasa, MD, PhD, Department of General and Molecular Pathology, University of Tartu, Tartu, Estonia Assistant Professor Oivi Uibo, MD, PhD, Department of Paediatrics, University of Tartu, Tartu, Estonia Opponent: Professor Lisbeth Tranebjærg, MD, PhD, Department of Audiology, H:S Bispebjerg Hospital, and Wilhelm Johannsen Centre of Functional Genomics Institute of Cellular and Molecular Medicine, ICMM, University of Copenhagen, The Panum Institute, Denmark Commencement: November 24, 2010 ISSN 1024–395x ISBN 978–9949–19–478–0 (trükis) ISBN 978–9949–19–479–7 (PDF) Autoriõigus: Rita Teek, 2010 Tartu Ülikooli Kirjastus www.tyk.ee Tellimuse nr. 570 To my patients and their families CONTENTS LIST OF ORIGINAL PUBLICATIONS ...................................................... 9 ABBREVIATIONS OF HEARING LOSS STUDY GROUPS AND PATIENTS .................................................................................................... -

NOISE and MILITARY SERVICE Implications for Hearing Loss and Tinnitus
NOISE AND MILITARY SERVICE Implications for Hearing Loss and Tinnitus Committee on Noise-Induced Hearing Loss and Tinnitus Associated with Military Service from World War II to the Present Medical Follow-up Agency Larry E. Humes, Lois M. Joellenbeck, and Jane S. Durch, Editors THE NATIONAL ACADEMIES PRESS Washington, DC www.nap.edu THE NATIONAL ACADEMIES PRESS • 500 Fifth Street, N.W. • Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This study was supported by Contract No. V101(93)P-1637 #29 between the Na- tional Academy of Sciences and the Department of Veterans Affairs. Any opinions, find- ings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the organizations or agencies that provided support for this project. Library of Congress Cataloging-in-Publication Data Noise and military service : implications for hearing loss and tinnitus / Committee on Noise-Induced Hearing Loss and Tinnitus Associated with Military Service from World War II to the Present, Medical Follow- up Agency ; Larry E. Humes, Lois M. Joellenbeck, and Jane S. Durch, editors. p. ; cm. Includes bibliographical references. ISBN 0-309-09949-8 — ISBN 0-309-65307-X 1. Deafness—Etiology. -

Hearing Screening Training Manual REVISED 12/2018
Hearing Screening Training Manual REVISED 12/2018 Minnesota Department of Health (MDH) Community and Family Health Division Maternal and Child Health Section 1 2 For more information, contact Minnesota Department of Health Maternal Child Health Section 85 E 7th Place St. Paul, MN 55164-0882 651-201-3760 [email protected] www.health.state.mn.us Upon request, this material will be made available in an alternative format such as large print, Braille or audio recording. 3 Revisions made to this manual are based on: Guidelines for Hearing Screening After the Newborn Period to Kindergarten Age http://www.improveehdi.org/mn/library/files/afternewbornperiodguidelines.pdf American Academy of Audiology, Childhood Screening Guidelines http://www.cdc.gov/ncbddd/hearingloss/documents/AAA_Childhood%20Hearing%2 0Guidelines_2011.pdf American Academy of Pediatrics (AAP), Hearing Assessment in Children: Recommendations Beyond Neonatal Screening http://pediatrics.aappublications.org/content/124/4/1252 4 Contents Introduction .................................................................................................................... 7 Audience ..................................................................................................................... 7 Purpose ....................................................................................................................... 7 Overview of hearing and hearing loss ............................................................................ 9 Sound, hearing, and hearing -
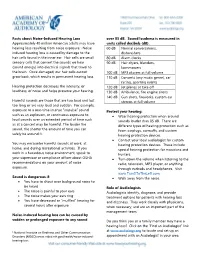
Facts About Noise-Induced Hearing Loss Over 85 Db
Facts about Noise-Induced Hearing Loss over 85 dB. Sound loudness is measured in Approximately 40 million American adults may have units called decibels (dB). hearing loss resulting from noise exposure.1 Noise- 60 dB Normal conversations, induced hearing loss is caused by damage to the dishwashers hair cells found in the inner ear. Hair cells are small 80 dB Alarm clocks sensory cells that convert the sounds we hear 90 dB Hair dryers, blenders, (sound energy) into electrical signals that travel to lawnmowers the brain. Once damaged, our hair cells cannot 100 dB MP3 players at full volume grow back, which results in permanent hearing loss. 110 dB Concerts (any music genre), car racing, sporting events Hearing protection decreases the intensity, or 120 dB Jet planes at take off loudness, of noise and helps preserve your hearing. 130 dB Ambulance, fire engine sirens 140 dB Gun shots, fireworks, custom car Harmful sounds are those that are too loud and last stereos at full volume too long or are very loud and sudden. For example, exposure to a one-time intense “impulse” sound Protect your hearing: such as an explosion, or continuous exposure to • Wear hearing protection when around loud sounds over an extended period of time such sounds louder than 85 dB. There are as at a concert may be harmful. The louder the different types of hearing protection such as sound, the shorter the amount of time you can foam earplugs, earmuffs, and custom safely be around it. hearing protection devices • Contact your local audiologist for custom You may encounter harmful sounds at work, at hearing protection devices. -

Deafness and Hearing Loss Caroline’S Story
Deafness and Hearing Loss Caroline’s Story Caroline is six years old, A publication of NICHCY with bright brown eyes and, at Disability Fact Sheet #3 June 2010 the moment, no front teeth, like so many other first graders. She also wears a hearing aid in each ear—and has done so since she was three, when she Caroline was immediately Hearing Loss was diagnosed with a moderate fitted with hearing aids. She in Children hearing loss. also began receiving special education and related services Hearing is one of our five For Caroline’s parents, there through the public school senses. Hearing gives us access were many clues along the way. system. Now in the first grade, to sounds in the world around Caroline often didn’t respond she regularly gets speech us—people’s voices, their to her name if her back was therapy and other services, and words, a car horn blown in turned. She didn’t startle at her speech has improved warning or as hello! noises that made other people dramatically. So has her vocabu- jump. She liked the TV on loud. lary and her attentiveness. She When a child has a hearing But it was the preschool she sits in the front row in class, an loss, it is cause for immediate started attending when she was accommodation that helps her attention. That’s because three that first put the clues hear the teacher clearly. She’s language and communication together and suggested to back on track, soaking up new skills develop most rapidly in Caroline’s parents that they information like a sponge, and childhood, especially before the have her hearing checked. -

Vestibular Neuritis, Labyrinthitis, and a Few Comments Regarding Sudden Sensorineural Hearing Loss Marcello Cherchi
Vestibular neuritis, labyrinthitis, and a few comments regarding sudden sensorineural hearing loss Marcello Cherchi §1: What are these diseases, how are they related, and what is their cause? §1.1: What is vestibular neuritis? Vestibular neuritis, also called vestibular neuronitis, was originally described by Margaret Ruth Dix and Charles Skinner Hallpike in 1952 (Dix and Hallpike 1952). It is currently suspected to be an inflammatory-mediated insult (damage) to the balance-related nerve (vestibular nerve) between the ear and the brain that manifests with abrupt-onset, severe dizziness that lasts days to weeks, and occasionally recurs. Although vestibular neuritis is usually regarded as a process affecting the vestibular nerve itself, damage restricted to the vestibule (balance components of the inner ear) would manifest clinically in a similar way, and might be termed “vestibulitis,” although that term is seldom applied (Izraeli, Rachmel et al. 1989). Thus, distinguishing between “vestibular neuritis” (inflammation of the vestibular nerve) and “vestibulitis” (inflammation of the balance-related components of the inner ear) would be difficult. §1.2: What is labyrinthitis? Labyrinthitis is currently suspected to be due to an inflammatory-mediated insult (damage) to both the “hearing component” (the cochlea) and the “balance component” (the semicircular canals and otolith organs) of the inner ear (labyrinth) itself. Labyrinthitis is sometimes also termed “vertigo with sudden hearing loss” (Pogson, Taylor et al. 2016, Kim, Choi et al. 2018) – and we will discuss sudden hearing loss further in a moment. Labyrinthitis usually manifests with severe dizziness (similar to vestibular neuritis) accompanied by ear symptoms on one side (typically hearing loss and tinnitus). -

Hearing Loss
Randal W. Swenson, M.D. Joshua G. Yorgason, M.D. David K. Palmer, M.D. Wesley R. Brown, M.D. John E. Butler, M.D. Nancy J. Stevenson, PA-C Justin D. Gull, M.D. ENT SPECIALISTS Kristin G. Hoopes, PA-C www.entslc.com Hearing Loss Approximately one in ten persons in the United may result from blockage of the ear canal (wax), States has some degree of hearing loss. Hearing is from a perforation (hole) in the ear drum, or from measured in decibels (dB), and a hearing level of 0- infection or disease of any of the three middle ear 25 dB is considered normal hearing. Your level is: bones. With a conductive loss only, the patient will never go deaf, but will always be able to hear, either Right ear _______ dB Left ear _______dB with reconstructive ear surgery or by use of a properly fitted hearing aid. Some patients who are Hearing Severity / % Loss not candidates for surgery, may benefit from a new 25 dB (normal).….0% 65dB(Severe)……...60% technology, the Baha (bone-anchored hearing aid). 35 dB (mild)……..15% 75dB(Severe)……...75% When there is a problem with the inner ear or 45 dB (moderate)..30% >85dB (Profound)..>90% nerve of hearing, a sensori-neural hearing loss occurs. This is most commonly from normal aging, Normal speech discrimination is 88-100%. Yours is: is usually worse in high frequencies, and can progress to total deafness. Noise exposure is another Right ear _______ % Left ear_______% common cause of high frequency hearing loss. Patients with sensori-neural hearing loss usually complain of difficulty hearing in loud environments. -

Sudden Bilateral Simultaneous Deafness with Vertigo As a Sole Manifestation of Vertebrobasilar Insufficiency H Lee, H a Yi, R W Baloh
539 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.4.539 on 1 April 2003. Downloaded from SHORT REPORT Sudden bilateral simultaneous deafness with vertigo as a sole manifestation of vertebrobasilar insufficiency H Lee, H A Yi, R W Baloh ............................................................................................................................. J Neurol Neurosurg Psychiatry 2003;74:539–541 CASE REPORT A 68 year old woman presented with bilateral sudden A 68 year old woman with long standing non-insulin depend- simultaneous hearing loss and transient spontaneous ent diabetes mellitus and hypertension suddenly developed vertigo as a sole manifestation of vertebrobasilar vertigo, vomiting, and bilateral tinnitus. Five minutes later, insufficiency. Extensive investigation to exclude other she noticed bilateral hearing loss. She had difficulty hearing causes was unremarkable. Magnetic resonance imaging her husband speak. She did not have dysarthria, numbness, of the brain, including diffusion images, showed no abnor- weakness, visual distortion, or incoordination. The hearing malities. A magnetic resonance angiogram showed severe loss persisted, but vertigo resolved over a few hours. Two days stenosis of the middle third of the basilar artery. A pure previously, she had had two episodes of transient vertigo and tone audiogram showed moderate sensorineural-type hearing loss involving the left ear that lasted no more than a hearing loss bilaterally. The localisation and mechanism of few minutes without any accompanying neurological symp- an isolated cochleovestibular dysfunction are discussed. toms. The patient had no previous history of hearing difficulty. There was no prior history of temporal bone fracture, menin- gitis, autoimmune disease, or exposure to ototoxic drugs. On admission, she had persistent bilateral hearing loss and ertebrobasilar insufficiency (VBI) can cause sudden non-specific dizziness with lightheadedness. -

Central Auditory Function Evaluation to Diagnose Central Auditory Processing Disorder Product Applicability
bmchp.org | 888-566-0008 wellsense.org | 877-957-1300 Medical Policy Central Auditory Function Evaluation to Diagnose Central Auditory Processing Disorder Policy Number: OCA 3.82 Version Number: 17 Version Effective Date: 01/01/21 + Product Applicability All Plan Products Well Sense Health Plan ∆ Boston Medical Center HealthNet Plan ∆ Well Sense Health Plan MassHealth ACO MassHealth MCO Qualified Health Plans/ConnectorCare/Employer Choice Direct Senior Care Options Notes: + Disclaimer and audit information is located at the end of this document. ∆ As required by state regulations and/or benefit coverage, different Plan definitions are specified for BMC HealthNet Plan products and Well Sense Health Plan products. See the applicable Definitions section for the Plan member. Policy Summary The evaluation of central auditory functioning to diagnose central auditory processing disorder (CAPD) is considered medically necessary for an adult or pediatric member when applicable Plan criteria are met. The evaluation is medically necessary when it is not part of a member’s individualized education plan (IEP) when one is appropriate for the member, it is a covered benefit for the member, and the Plan’s medical criteria are met (as specified in the Medical Policy Statement section of this Plan policy). Prior authorization is required for Plan covered services. See the member’s applicable benefit document available at www.bmchp.org for a member enrolled in a BMC HealthNet Plan product or Central Auditory Function Evaluation to Diagnose Central Auditory Processing Disorder + Plan refers to Boston Medical Center Health Plan, Inc. and its affiliates and subsidiaries offering health coverage plans to enrolled members. -

Hearing Impairment Sheet
Oklahoma State Department of Education Special Education Services • 405-521-3351 • www.ok.gov/sde/special-education FACT HEARING IMPAIRMENT SHEET ■ Definition of Intellectual POSSIBLE CAUSES Disabilities under IDEA • Acquired, meaning that the loss occurred after birth, Hearing impairment means an impairment in hearing, due to illness or injury; or whether permanent or fluctuating, that adversely affects • Congenital, meaning that the hearing loss or deafness a child’s educational performance. 34 CFR 300.8(c)(5) was present at birth The most common cause of acquired hearing loss is TYPES exposure to noise (Merck Manual’s Online Medical Conductive hearing losses are caused by diseases or Library, 2007). Other causes can include: obstructions in the outer or middle ear (the pathways • Build up of fluid behind the eardrum for sound to reach the inner ear). Conductive hearing • Ear infections (known as otitis media) losses usually affect all frequencies of hearing evenly • Childhood diseases, such as mumps, measles, or and do not result in severe losses. A person with a con- chicken pox; and ductive hearing loss usually is able to use a hearing aid • Head trauma well or can be helped medically or surgically. Congenital causes of hearing loss and Sensorineural hearing losses result from damage to the deafness include: delicate sensory hair cells of the inner ear or the nerves • A family history of hearing loss or deafness that supply it. These hearing losses can range from • Infections during pregnancy (such as rubella) mild to profound. They often affect the person’s abil- • Complications during pregnancy (such as the Rh ity to hear certain frequencies more than others.