Murine Neonatal Ketogenesis Preserves Mitochondrial Energetics by Preventing Protein Hyperacetylation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upregulation of Peroxisome Proliferator-Activated Receptor-Α And
Upregulation of peroxisome proliferator-activated receptor-α and the lipid metabolism pathway promotes carcinogenesis of ampullary cancer Chih-Yang Wang, Ying-Jui Chao, Yi-Ling Chen, Tzu-Wen Wang, Nam Nhut Phan, Hui-Ping Hsu, Yan-Shen Shan, Ming-Derg Lai 1 Supplementary Table 1. Demographics and clinical outcomes of five patients with ampullary cancer Time of Tumor Time to Age Differentia survival/ Sex Staging size Morphology Recurrence recurrence Condition (years) tion expired (cm) (months) (months) T2N0, 51 F 211 Polypoid Unknown No -- Survived 193 stage Ib T2N0, 2.41.5 58 F Mixed Good Yes 14 Expired 17 stage Ib 0.6 T3N0, 4.53.5 68 M Polypoid Good No -- Survived 162 stage IIA 1.2 T3N0, 66 M 110.8 Ulcerative Good Yes 64 Expired 227 stage IIA T3N0, 60 M 21.81 Mixed Moderate Yes 5.6 Expired 16.7 stage IIA 2 Supplementary Table 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of an ampullary cancer microarray using the Database for Annotation, Visualization and Integrated Discovery (DAVID). This table contains only pathways with p values that ranged 0.0001~0.05. KEGG Pathway p value Genes Pentose and 1.50E-04 UGT1A6, CRYL1, UGT1A8, AKR1B1, UGT2B11, UGT2A3, glucuronate UGT2B10, UGT2B7, XYLB interconversions Drug metabolism 1.63E-04 CYP3A4, XDH, UGT1A6, CYP3A5, CES2, CYP3A7, UGT1A8, NAT2, UGT2B11, DPYD, UGT2A3, UGT2B10, UGT2B7 Maturity-onset 2.43E-04 HNF1A, HNF4A, SLC2A2, PKLR, NEUROD1, HNF4G, diabetes of the PDX1, NR5A2, NKX2-2 young Starch and sucrose 6.03E-04 GBA3, UGT1A6, G6PC, UGT1A8, ENPP3, MGAM, SI, metabolism -

KAT5 Promotes Invasion and Metastasis Through C-MYC Stabilization in ATC
26 1 Endocrine-Related X Wei, S Cai et al. KAT5 in anaplastic thyroid 26:1 141–151 Cancer carcinoma RESEARCH KAT5 promotes invasion and metastasis through C-MYC stabilization in ATC Xi Wei1,*, Shang Cai2,3,*, Rebecca J Boohaker2, Joshua Fried2, Ying Li4, Linfei Hu5, Yi Pan5, Ruifen Cheng5, Sheng Zhang1, Ye Tian3, Ming Gao5 and Bo Xu2,6 1Department of Diagnostic and Therapeutic Ultrasonography, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, China 2Department of Oncology, Southern Research Institute and Cancer Cell Biology Program, the University of Alabama at Birmingham Comprehensive Cancer Center, Birmingham, Alabama, USA 3Department of Radiotherapy and Oncology, the Second Affiliated Hospital ofoochow S University, Suzhou, China 4The Third Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, China 5Department of Thyroid Tumor, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, China 6Department of Molecular Radiation Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, Ministry of Education, National Clinical Research Center of Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China Correspondence should be addressed to B Xu or M Gao: [email protected] or [email protected] or [email protected] *(X Wei and S Cai contributed equally to this work) Abstract Anaplastic thyroid cancer (ATC) is an aggressive cancer with poor clinical prognosis. Key Words However, mechanisms driving ATC aggressiveness is not well known. -

Electronic Supplementary Material (ESI) for Molecular Biosystems
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is © The Royal Society of Chemistry 2015 Table S5 Mass data of proteins identified with label free shotgun proteomics a #Alt. Proteins; b Scores; c #Peptides; d SC [%]; e RMS90 [ppm]; f Rank; g Median(Controls:GLP1); h #(Controls:GLP1); i CV [%](Controls:GLP1); l Median(Controls:Palmitate); m #(Controls:Palmitate) ; n CV [%](Controls:Palmitate); o Median(Controls:GLP1 + Palmitate); p #(Controls:GLP1 + Palmitate); q CV [%](Controls:GLP1 + Palmitate) MW OK Accession Protein pI a b c d e f g h i l m n o p q [kDa] 78 kDa glucose-regulated protein OS=Rattus 1672.6 true GRP78_RAT 72.3 4.9 1 22 39 1.48 1 1.16 6 20.53 1.18 9 9.56 1.24 10 13.52 norvegicus GN=Hspa5 PE=1 SV=1 (M:1672.6) Endoplasmin OS=Rattus norvegicus 1253.0 true ENPL_RAT 92.7 4.6 1 23 29 3.34 2 0.85 8 33.34 1.19 6 39.58 1.18 10 28.11 GN=Hsp90b1 PE=1 SV=2 (M:1253.0) Stress-70 protein, mitochondrial OS=Rattus 1138.8 true GRP75_RAT 73.8 5.9 1 17 28.7 1.18 3 1.21 5 7.62 0.78 1 1.1 8 8.04 norvegicus GN=Hspa9 PE=1 SV=3 (M:1138.8) Protein disulfide-isomerase A3 OS=Rattus 1035.4 true PDIA3_RAT 56.6 5.8 1 16 35.2 2.84 4 0.94 3 17.22 1.15 4 26.62 1.25 4 7.86 norvegicus GN=Pdia3 PE=1 SV=2 (M:1035.4) Aconitate hydratase, mitochondrial 983.8 true ACON_RAT OS=Rattus norvegicus GN=Aco2 PE=1 85.4 8.7 1 19 31.4 2.72 5 0.89 2 23.6 1.21 2 9.25 0.89 3 32.87 (M:983.8) SV=2 60 kDa heat shock protein, mitochondrial 906.9 true CH60_RAT OS=Rattus norvegicus GN=Hspd1 PE=1 60.9 5.8 1 13 32.5 3.26 6 1.05 5 13.15 1.01 2 32.54 1.01 -

4-6 Weeks Old Female C57BL/6 Mice Obtained from Jackson Labs Were Used for Cell Isolation
Methods Mice: 4-6 weeks old female C57BL/6 mice obtained from Jackson labs were used for cell isolation. Female Foxp3-IRES-GFP reporter mice (1), backcrossed to B6/C57 background for 10 generations, were used for the isolation of naïve CD4 and naïve CD8 cells for the RNAseq experiments. The mice were housed in pathogen-free animal facility in the La Jolla Institute for Allergy and Immunology and were used according to protocols approved by the Institutional Animal Care and use Committee. Preparation of cells: Subsets of thymocytes were isolated by cell sorting as previously described (2), after cell surface staining using CD4 (GK1.5), CD8 (53-6.7), CD3ε (145- 2C11), CD24 (M1/69) (all from Biolegend). DP cells: CD4+CD8 int/hi; CD4 SP cells: CD4CD3 hi, CD24 int/lo; CD8 SP cells: CD8 int/hi CD4 CD3 hi, CD24 int/lo (Fig S2). Peripheral subsets were isolated after pooling spleen and lymph nodes. T cells were enriched by negative isolation using Dynabeads (Dynabeads untouched mouse T cells, 11413D, Invitrogen). After surface staining for CD4 (GK1.5), CD8 (53-6.7), CD62L (MEL-14), CD25 (PC61) and CD44 (IM7), naïve CD4+CD62L hiCD25-CD44lo and naïve CD8+CD62L hiCD25-CD44lo were obtained by sorting (BD FACS Aria). Additionally, for the RNAseq experiments, CD4 and CD8 naïve cells were isolated by sorting T cells from the Foxp3- IRES-GFP mice: CD4+CD62LhiCD25–CD44lo GFP(FOXP3)– and CD8+CD62LhiCD25– CD44lo GFP(FOXP3)– (antibodies were from Biolegend). In some cases, naïve CD4 cells were cultured in vitro under Th1 or Th2 polarizing conditions (3, 4). -

KAT5 Acetylates Cgas to Promote Innate Immune Response to DNA Virus
KAT5 acetylates cGAS to promote innate immune response to DNA virus Ze-Min Songa, Heng Lina, Xue-Mei Yia, Wei Guoa, Ming-Ming Hua, and Hong-Bing Shua,1 aDepartment of Infectious Diseases, Zhongnan Hospital of Wuhan University, Frontier Science Center for Immunology and Metabolism, Medical Research Institute, Wuhan University, 430071 Wuhan, China Edited by Adolfo Garcia-Sastre, Icahn School of Medicine at Mount Sinai, New York, NY, and approved July 30, 2020 (received for review December 19, 2019) The DNA sensor cGMP-AMP synthase (cGAS) senses cytosolic mi- suppress its enzymatic activity (15). It has also been shown that crobial or self DNA to initiate a MITA/STING-dependent innate im- the NUD of cGAS is critically involved in its optimal DNA- mune response. cGAS is regulated by various posttranslational binding (16), phase-separation (7), and subcellular locations modifications at its C-terminal catalytic domain. Whether and (17). However, whether and how the NUD of cGAS is regulated how its N-terminal unstructured domain is regulated by posttrans- remains unknown. lational modifications remain unknown. We identified the acetyl- The lysine acetyltransferase 5 (KAT5) is a catalytic subunit of transferase KAT5 as a positive regulator of cGAS-mediated innate the highly conserved NuA4 acetyltransferase complex, which immune signaling. Overexpression of KAT5 potentiated viral- plays critical roles in DNA damage repair, p53-mediated apo- DNA–triggered transcription of downstream antiviral genes, whereas ptosis, HIV-1 transcription, and autophagy (18–21). Although a KAT5 deficiency had the opposite effects. Mice with inactivated KAT5 has been investigated mostly as a transcriptional regula- Kat5 exhibited lower levels of serum cytokines in response to DNA tor, there is increasing evidence that KAT5 also acts as a key virus infection, higher viral titers in the brains, and more susceptibility regulator in signal transduction pathways by targeting nonhis- to DNA-virus–induced death. -

Atrazine and Cell Death Symbol Synonym(S)
Supplementary Table S1: Atrazine and Cell Death Symbol Synonym(s) Entrez Gene Name Location Family AR AIS, Andr, androgen receptor androgen receptor Nucleus ligand- dependent nuclear receptor atrazine 1,3,5-triazine-2,4-diamine Other chemical toxicant beta-estradiol (8R,9S,13S,14S,17S)-13-methyl- Other chemical - 6,7,8,9,11,12,14,15,16,17- endogenous decahydrocyclopenta[a]phenanthrene- mammalian 3,17-diol CGB (includes beta HCG5, CGB3, CGB5, CGB7, chorionic gonadotropin, beta Extracellular other others) CGB8, chorionic gonadotropin polypeptide Space CLEC11A AW457320, C-type lectin domain C-type lectin domain family 11, Extracellular growth factor family 11, member A, STEM CELL member A Space GROWTH FACTOR CYP11A1 CHOLESTEROL SIDE-CHAIN cytochrome P450, family 11, Cytoplasm enzyme CLEAVAGE ENZYME subfamily A, polypeptide 1 CYP19A1 Ar, ArKO, ARO, ARO1, Aromatase cytochrome P450, family 19, Cytoplasm enzyme subfamily A, polypeptide 1 ESR1 AA420328, Alpha estrogen receptor,(α) estrogen receptor 1 Nucleus ligand- dependent nuclear receptor estrogen C18 steroids, oestrogen Other chemical drug estrogen receptor ER, ESR, ESR1/2, esr1/esr2 Nucleus group estrone (8R,9S,13S,14S)-3-hydroxy-13-methyl- Other chemical - 7,8,9,11,12,14,15,16-octahydro-6H- endogenous cyclopenta[a]phenanthren-17-one mammalian G6PD BOS 25472, G28A, G6PD1, G6PDX, glucose-6-phosphate Cytoplasm enzyme Glucose-6-P Dehydrogenase dehydrogenase GATA4 ASD2, GATA binding protein 4, GATA binding protein 4 Nucleus transcription TACHD, TOF, VSD1 regulator GHRHR growth hormone releasing -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Sponges Are Highly Resistant to Radiation Exposure and Cancer
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.17.435910; this version posted March 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Sponges are highly resistant to radiation exposure and cancer Angelo Fortunato1,2,3†, Jake Taylor1,2,3, Jonathan Scirone1,2,3, Athena Aktipis1,4* and Carlo C. Maley1,2,3* 1. Arizona Cancer Evolution Center, Arizona State University, 1001 S. McAllister Ave., Tempe, AZ, 85287, USA. 2. Biodesign Center for Biocomputing, Security and Society, Arizona State University, 727 E. Tyler St.,Tempe, AZ 85281, USA. 3. School of Life Sciences, Arizona State University, 427 East Tyler Mall, Tempe, AZ 85287, USA. 4. Department of Psychology, Arizona State University, Tempe, AZ, USA. † Corresponding author * co-senior authors bioRxiv preprint doi: https://doi.org/10.1101/2021.03.17.435910; this version posted March 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract There are no reports of cancer in sponges, despite them having somatic cell turnover, long lifespans and no specialized adaptive immune cells. In order to investigate whether sponges are cancer resistant, we exposed a species of sponge, Tethya wilhelma, to X-rays. We found that T. wilhelma can withstand 600 Gy of X-ray radiation. That is approximately 100 times the lethal dose for humans. A single high dose of X-rays did not induce cancer in sponges, providing the first experimental evidence of cancer resistance in the phylum, Porifera. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

New Mechanisms That Regulate the Expression of Genes Implicated in the Process of Ketogenesis
Isabel Alexandra Pinto Carrilho do Rosário Licenciatura em Bioquímica New mechanisms that regulate the expression of genes implicated in the process of ketogenesis Dissertação para obtenção do Grau de Mestre em Biotecnologia Orientador: Prof. Dr. Pedro F. Marrero González, Prof. Titular, Facultat de Farmàcia, Universitat de Barcelona Co-orientador: Prof. Dr. Diego Haro Bautista, Prof. Catedrático, Facultat de Farmàcia, Universitat de Barcelona Presidente: Prof. Doutora Isabel Maria Godinho de Sá Nogueira Arguente: Prof. Doutor Pedro Miguel Ribeiro Viana Baptista Setembro, 2012 Isabel Alexandra Pinto Carrilho do Rosário Licenciatura em Bioquímica New mechanisms that regulate the expression of genes implicated in the process of ketogenesis Dissertação para obtenção do Grau de Mestre em Biotecnologia Orientador: Prof. Dr. Pedro F. Marrero González, Prof. Titular, Facultat de Farmàcia, Universitat de Barcelona Co-orientador: Prof. Dr. Diego Haro Bautista, Prof. Catedrático, Facultat de Farmàcia, Universitat de Barcelona Setembro, 2012 Copyright New mechanisms that regulate the expression of genes implicated in the process of ketogenesis © Isabel Alexandra Pinto Carrilho do Rosário FCT/UNL UNL A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição, com objectivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. i ii Ninguém sabe que coisa quer. Ninguém conhece que alma tem, Nem o que é mal nem o que o bem. -
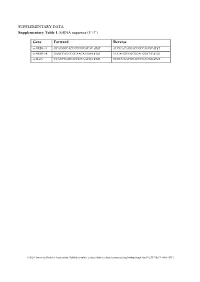
Supplementary Tables and Figures
SUPPLEMENTARY DATA Supplementary Table 1. SiRNA sequence (5’-3’) Gene Forward Reverse si-HRD1-1# GCAUGGCAGUCCUGUACAU dTdT AUGUACAGGACUGCCAUGC dTdT si-HRD1-2# GAGCCAUCCGCAACAUGAA dTdT UUCAUGUUGCGGAUGGCUC dTdT si-MafA CCAUCGAGUACGUCAACGA dTdT UCGUUGACGUACUCGAUGG dTdT ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1060/-/DC1 SUPPLEMENTARY DATA Supplementary Table 2. Primer sequences for qRT-PCR (5’-3’) Gene Forward Reverse human HRD1 GCTCACGCCTACTACCTCAAA GCCAGACAAGTCTCTGTGACG mouse mafA AAGCGGCGCACGCTCAAGAA GGTCCCGCTCCTTGGCCAGA mouse insulin1 CACTTCCTACCCCTGCTGG ACCACAAAGATGCTGTTTGACA mouse β-actin AGGCCAACCGTGAAAAGATG AGAGCATAGCCCTCGTAGATGG human β-actin CATGTACGTTGCTATCCAGGC CTCCTTAATGTCACGCACGAT ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1060/-/DC1 SUPPLEMENTARY DATA Supplementary Table 3. Primer sequences for ChIP (5’-3’) Gene promoter Forward Reverse mouse Insulin1, 2 GGAACTGTGAAACAGTCCAAGG CCCCCTGGACTTTGCTGTTTG ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1060/-/DC1 SUPPLEMENTARY DATA Supplementary Table 4. Primer sequences for PCR (5’-3’) Gene Forward Reverse HRD1-pDsred CCCAAGCTTATGTTCCGCACCGCAGT GGGGTACCCAGTGGGCAACAGGGG HRD1-pCMV- Flag GGGGTACCATGTTCCGCACCGCAGT CCCAAGCTTGTGGGCAACAGGGGACT C HRD1-pCMV-HA GGCCATGGGCCATATGGGATCCTTCC AGGGATGCCACCCGGGGATCCTCAGT GCACCGCAGTGATG GGGCAACAGGGGAC HRD1-N-HA GGCCATGGGCCATATGGGATCCTTCC -

Dissertation
Mechanism and dynamics of acyl-CoA mediated protein lysine acylation in mitochondria Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Naturwissenschaftlichen Fakultät I – Biowissenschaften – der Martin-Luther-Universität Halle Wittenberg, vorgelegt von Herrn Zeljko Simic geb. am 04.02.1982 in Bajina Basta Gutachter: Prof. Dr. Mike Schutkowski Prof. Dr. Wolfgang Sippl Prof. Dr. Dirk Schwarzer Tag der öffentlichen Verteidigung: 13.10.2016 Table of contents Abbreviations list ....................................................................................................................................... iv List of figures ............................................................................................................................................ viii List of tables ............................................................................................................................................... xi Introduction .................................................................................................................................................. 1 Lysine acetylation ................................................................................................................................... 1 Protein lysine acetylation in mitochondria .......................................................................................... 3 Induction of mitochondrial protein hyperacetylation .......................................................................... 5