A New Reconstruction of Multituberculate Endocranial Casts and Encephalization Quotient of Kryptobaatar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dome-Headed, Small-Brained Island Mammal from the Late Cretaceous of Romania
Dome-headed, small-brained island mammal from the Late Cretaceous of Romania Zoltán Csiki-Savaa,1, Mátyás Vremirb, Jin Mengc, Stephen L. Brusatted, and Mark A. Norellc aLaboratory of Paleontology, Faculty of Geology and Geophysics, University of Bucharest, 010041 Bucharest, Romania; bDepartment of Natural Sciences, Transylvanian Museum Society, 400009 Cluj-Napoca, Romania; cDivision of Paleontology, American Museum of Natural History, New York, NY 10024; and dSchool of GeoSciences, Grant Institute, University of Edinburgh, EH9 3FE Edinburgh, United Kingdom Edited by Neil H. Shubin, The University of Chicago, Chicago, IL, and approved March 26, 2018 (received for review January 20, 2018) The island effect is a well-known evolutionary phenomenon, in describe the anatomy of kogaionids in detail, include them in a which island-dwelling species isolated in a resource-limited envi- comprehensive phylogenetic analysis, estimate their body sizes, ronment often modify their size, anatomy, and behaviors compared and present a reconstruction of their brain and sense organs. with mainland relatives. This has been well documented in modern This species exhibits several features that we interpret as re- and Cenozoic mammals, but it remains unclear whether older, more lated to its insular habitat, most notably a brain that is sub- primitive Mesozoic mammals responded in similar ways to island stantially reduced in size compared with close relatives and habitats. We describe a reasonably complete and well-preserved skeleton of a kogaionid, an enigmatic radiation of Cretaceous island- mainland contemporaries, demonstrating that some Mesozoic dwelling multituberculate mammals previously represented by frag- mammals were susceptible to the island effect like in more mentary fossils. -

Functional Tests of the Competitive Exclusion Hypothesis For
Functional tests of the competitive exclusion royalsocietypublishing.org/journal/rsos hypothesis for multituberculate extinction Research Cite this article: Adams NF, Rayfield EJ, Cox PG, Neil F. Adams1,†, Emily J. Rayfield1, Philip G. Cox2,3, Cobb SN, Corfe IJ. 2019 Functional tests of the 2,3 4 competitive exclusion hypothesis for Samuel N. Cobb and Ian J. Corfe multituberculate extinction. R. Soc. open sci. 6: 1School of Earth Sciences, University of Bristol, Bristol BS8 1RJ, UK 181536. 2Department of Archaeology, University of York, York YO1 7EP, UK http://dx.doi.org/10.1098/rsos.181536 3Centre for Anatomical and Human Sciences, Hull York Medical School, University of York, York YO10 5DD, UK 4Institute of Biotechnology, University of Helsinki, 00014 Helsinki, Finland NFA, 0000-0003-2539-5531; EJR, 0000-0002-2618-750X; Received: 11 September 2018 PGC, 0000-0001-9782-2358; SNC, 0000-0002-8360-8024; Accepted: 21 February 2019 IJC, 0000-0002-1824-755X Multituberculate mammals thrived during the Mesozoic, but their diversity declined from the mid-late Paleocene Subject Category: onwards, becoming extinct in the late Eocene. The radiation of Biology (whole organism) superficially similar, eutherian rodents has been linked to multituberculate extinction through competitive exclusion. Subject Areas: However, characteristics providing rodents with a supposed palaeontology/evolution/biomechanics competitive advantage are currently unknown and comparative functional tests between the two groups are lacking. Here, a Keywords: multifaceted approach -

Craniodental Anatomy of a New Late Cretaceous Multituberculate Mammal from Udan Sayr, Mongolia
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 8-2014 Craniodental anatomy of a new late cretaceous multituberculate mammal from Udan Sayr, Mongolia. Amir Subhash Sheth University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/etd Part of the Anatomy Commons, and the Medical Neurobiology Commons Recommended Citation Sheth, Amir Subhash, "Craniodental anatomy of a new late cretaceous multituberculate mammal from Udan Sayr, Mongolia." (2014). Electronic Theses and Dissertations. Paper 1317. https://doi.org/10.18297/etd/1317 This Master's Thesis is brought to you for free and open access by ThinkIR: The nivU ersity of Louisville's Institutional Repository. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of ThinkIR: The nivU ersity of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. CRANIODENTAL ANATOMY OF A NEW LATE CRETACEOUS MULTITUBERCULATE MAMMAL FROM UDAN SAYR, MONGOLIA By Amir Subhash Sheth B.A., Centre College, 2010 A Thesis Submitted to the Faculty of the School of Medicine of the University of Louisville in Partial Fulfillment of the Requirements for the Degree of Master of Science Department of Anatomical Sciences and Neurobiology University of Louisville Louisville, Kentucky August 2014 CRANIODENTAL ANATOMY OF A NEW LATE CRETACEOUS MULTITUBERCULATE MAMMAL FROM UDAN SAYR, MONGOLIA By Amir Subhash Sheth B.A., Centre College, 2010 A Thesis Approved on July 18th, 2014 By the Following Thesis Committee: ________________________________ (Guillermo W. -

Mammals from the Mesozoic of Mongolia
Mammals from the Mesozoic of Mongolia Introduction and Simpson (1926) dcscrihed these as placental (eutherian) insectivores. 'l'he deltathcroids originally Mongolia produces one of the world's most extraordi- included with the insectivores, more recently have narily preserved assemblages of hlesozoic ma~nmals. t)een assigned to the Metatheria (Kielan-Jaworowska Unlike fossils at most Mesozoic sites, Inany of these and Nesov, 1990). For ahout 40 years these were the remains are skulls, and in some cases these are asso- only Mesozoic ~nanimalsknown from Mongolia. ciated with postcranial skeletons. Ry contrast, 'I'he next discoveries in Mongolia were made by the Mesozoic mammals at well-known sites in North Polish-Mongolian Palaeontological Expeditions America and other continents have produced less (1963-1971) initially led by Naydin Dovchin, then by complete material, usually incomplete jaws with den- Rinchen Barsbold on the Mongolian side, and Zofia titions, or isolated teeth. In addition to the rich Kielan-Jaworowska on the Polish side, Kazi~nierz samples of skulls and skeletons representing Late Koualski led the expedition in 1964. Late Cretaceous Cretaceous mam~nals,certain localities in Mongolia ma~nmalswere collected in three Gohi Desert regions: are also known for less well preserved, but important, Bayan Zag (Djadokhta Formation), Nenlegt and remains of Early Cretaceous mammals. The mammals Khulsan in the Nemegt Valley (Baruungoyot from hoth Early and Late Cretaceous intervals have Formation), and llcrmiin 'ISav, south-\vest of the increased our understanding of diversification and Neniegt Valley, in the Red beds of Hermiin 'rsav, morphologic variation in archaic mammals. which have heen regarded as a stratigraphic ecluivalent Potentially this new information has hearing on the of the Baruungoyot Formation (Gradzinslti r't crl., phylogenetic relationships among major branches of 1977). -

AMERICAN MUSEUM NOVITATES Published by Number 330 Thz Auerican Museum of NAITURAL HISTORY October 30, 1928 New York City
AMERICAN MUSEUM NOVITATES Published by Number 330 THz AuERicAN MusEUM oF NAITURAL HISTORY October 30, 1928 New York City 56.9,33 (117:51.7) AFFINITIES OF THE MONGOLIAN CRETACEOUS INSECTIVORES' BY GEORGE GAYLORD SIMPSON The unique series of Mesozoic mammal remains found by the Third Asiatic Expedition in Mongolia has now been completely de- scribed in a series of three papers.2 The affinities of the one known multituberculate, Djadochtatherium matthewi, were as thoroughly discussed as the material warrants in the first paper, and no additional remarks seem necessary. The relationships of the more important insectivores, however, were only briefly discussed in the second paper and a review of the evidence, especially including the important new details given in the third paper, suggests some modification and amplification of the views already presented. Not only are these mammal remains by far the most complete ever discovered in the Mesozoic, but they also occupy a very strategic position in time and in space which makes close scrutiny of their relationships essential. In time they occur in the Cretaceous, when, according to theories formed before their discovery and based largely on early Tertiary mammals, the differentiation of the placental orders should be in progress and not yet far advanced. In space they occur in Central Asia in or near the region which a number of students, especially Osborn and Matthew, have considered as an important center of radiation and probably the very one whence came the groups of mammals which appear to have entered North America and Europe suddenly at the beginning of the Tertiary and which must have been undergoing an important deployment during upper Cretaceous time. -
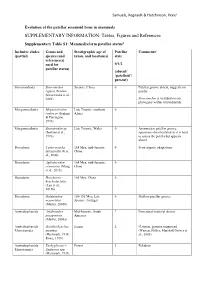
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

The Braincase of Two Late Cretaceous Asian Multituberculates Studied by Serial Sections
The braincase of two Late Cretaceous Asian multituberculates studied by serial sections J0RN H. HURUM Hurum, J.H. 1998. The braincase of two Late Cretaceous Asian multituberculatesstudied by serial sections. -Acta Palaeontologica Polonica 43, 1, 21-52. The braincase structure of two Late Cretaceous Mongolian djadochtatherian multituber- culates Nemegtbaatar gobiensis and Chulsanbaatar vulgaris from the ?late Campanian of Mongolia is presented based on the two serially sectioned skulls and additional specimens. Reconstructions of the floor of the braincase in both taxa are given. The complete intracranial sphenoid region is reconstructed for the first time in multitubercu- lates. Cavum epiptericum is a separate space with the taenia clino-orbitalis (ossified pila antotica) as the medial wall, anterior lamina of the petrosal and possibly the alisphenoid as the lateral wall, and the basisphenoid, petrosal and possibly alisphenoid ventrally. The fovea hypochiasmatica is shallow, tuberculurn sellae is wide and more raised from the skull base than it is in the genus Pseudobolodon. The dorsal opening of the carotid canal is situated in the fossa hypophyseos. The taenia clino-orbitalis differs from the one described in Pseudobolodon and Lambdopsalis in possessing just one foramen (metoptic foramen). Compared to all extant mammals the braincase in Nemegtbaatar and Chulsan- baatar is primitive in that both the pila antotica and pila metoptica are retained. In both genera the anterior lamina of the petrosal is large with a long anterodorsal process while the alisphenoid is small. A review is given of the cranial anatomy in Nemegtbaatar and Chulsanbaatar. K e y w o r d s : Braincase structure, sphenoid complex, cavum epiptericum,Mammalia, Multituberculata,Djadochtatheria, Cretaceous, Mongolia. -

New Data on the Skull and Dentition in the Mongolian Late Cretaceous Eutherian Mammal Zalambdalestes
NEW DATA ON THE SKULL AND DENTITION IN THE MONGOLIAN LATE CRETACEOUS EUTHERIAN MAMMAL ZALAMBDALESTES JOHN R. WIBLE Section of Mammals, Carnegie Museum of Natural History, 5800 Baum Boulevard, Pittsburgh, PA 15206 e-mail: [email protected] MICHAEL J. NOVACEK Division of Paleontology, American Museum of Natural History e-mail: [email protected] GUILLERMO W. ROUGIER Department of Anatomical Sciences and Neurobiology, School of Medicine, University of Louisville, Louisville, KY 40292 e-mail: [email protected] BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 281, 144 pp., 58 ®gures, 3 tables Issued January 9, 2004 Copyright q American Museum of Natural History 2004 ISSN 0003-0090 2 BULLETIN AMERICAN MUSEUM OF NATURAL HISTORY NO. 281 CONTENTS Abstract ....................................................................... 3 Introduction .................................................................... 3 Materials and Methods .......................................................... 5 History of Investigations ......................................................... 7 Comparative Morphology ....................................................... 17 Dentition ................................................................... 17 Upper Incisors ............................................................ 18 Upper Canine ............................................................. 21 Upper Premolars .......................................................... 22 Upper -

Multituberculate Mammals from the Cretaceous of Uzbekistan
Multituberculate mammals from the Cretaceous of Uzbekistan ZOFIA KIELAN- JAWOROWSKA and LEV A. NESSOV Kielan-Jaworowska, 2. & Nessov, L. A. 1992. Multituberculate mammals from the Cretaceous of Uzbelustan. Acta Palaeontologica Polonica 37, 1, 1- 17. The first western Asian multituberculates found in the Bissekty Formation (Co- niacian) of Uzbekistan are described on the basis of a lower premolar (p4), a fragment of a lower incisor, an edentulous dentary, a proximal part of the humerus and a proximal part of the femur. Uzbekbaatar kizylkumensis gen. et sp. n. is defined as having a low and arcuate p4. possibly without a posterobuccal cusp; it presumably had two lower premolars, as inferred from the presence of a triangular concavity at the upper part of the anterior wall of p4, and p3 less reduced in relation to p4 than in non-specialized Taeniolabidoidea and Ptilodontoidea. Uzbek- baatar is placed in the Cimolodonta without indicating family and infraorder. It might have originated from the Plagiaulacinae or Eobaatarinae. Key words : Multituberculata, Marnmalia, Cretaceous, Coniacian, Uzbekistan. Zofia Kielan-Jaworowska. Paleontologisk Museum, Universitetet i Oslo, Sars Gate 1, N-0562 Oslo, Norway. flec A. Hecoc, kf~cmumym3exnoiL Kopbl, Ca~~m-nemep6ypzc~uiiYnueepcumem, 199 034 Ca~~m-l7emep6ypz,Poccun (Lev A. Nessov, Institute of the Earth Crust, Sankt-Peters- burg University, 199 034 St. Petersburg. Russla]. Introduction The Multituberculata is the first mammalian order to have adapted to herbivorous niches, although many may have been omnivorous (Krause 1982). Known from the Late Triassic to the Early Oligocene (Hahn & Hahn 1983), this order of mammals was dominant throughout the Mesozoic in most of the local faunas studied. -

The Earliest Mammal of the European Paleocene: the Multituberculate Hainina
J. Paleont., 74(4), 2000, pp. 701–711 Copyright ᭧ 2000, The Paleontological Society 0022-3360/00/0074-0701$03.00 THE EARLIEST MAMMAL OF THE EUROPEAN PALEOCENE: THE MULTITUBERCULATE HAININA P. PELA´ EZ-CAMPOMANES,1 N. LO´ PEZ-MARTI´NEZ,2 M.A. A´ LVAREZ-SIERRA,2 R. DAAMS3 1Department Paleobiologı´a,Museo Nacional de Ciencias Naturales, Gutie´rrez Abascal 2, 28006 Madrid, Spain, Ͻ[email protected]Ͼ, and 2Department-UEI Paleontologı´a, Facultad C. Geolo´gicas-Instit. Geologı´a Econo´mica,Universidad Complutense-CSIC, 28040 Madrid, Spain ABSTRACT—A new species of multituberculate mammal, Hainina pyrenaica n. sp. is described from Fontllonga-3 (Tremp Basin, Southern Pyrenees, Spain), correlated to the later part of chron C29r just above the K/T boundary. This taxon represents the earliest European Tertiary mammal recovered so far, and is related to other Hainina species from the European Paleocene. A revision of the species of Hainina allows recognition of a new species, H. vianeyae n. sp. from the Late Paleocene of Cernay (France). The genus is included in the family Kogaionidae Ra˜dulescu and Samson, 1996 from the Late Cretaceous of Romania on the basis of unique dental characters. The Kogaionidae had a peculiar masticatory system with a large, blade-like lower p4, similar to that of advanced Ptilodon- toidea, but occluding against two small upper premolars, interpreted as P4 and P5, instead of a large upper P4. The endemic European Kogaionidae derive from an Early Cretaceous group with five premolars, and evolved during the Late Cretaceous and Paleocene. The genus Hainina represents a European multituberculate family that survived the K/T boundary mass extinction event. -

Masticatory Musculature of Asian Taeniolabidoid Multituberculate Mammals
Masticatory musculature of Asian taeniolabidoid multituberculate mammals PETR P. GAMBARYAN & ZOFIA KIELAN-JAWOROWSKA* Gambaryan, P.P. & Kielan-Jaworowska, 2. 1995. Masticatory musculature of Asian taeniolabidoid multituberculate mammals. Acta Palaeontologica Polonica 40, 1, 45-108. The backward chewing stroke in multituberculates (unique for mammals) resulted in a more anterior insertion of the masticatory muscles than in any other mammal group, including rodents. Multituberculates differ from tritylodontids in details of the masticatory musculature, but share with them the backward masticatory power stroke and retractory horizontal components of the resultant force of all the masticatory muscles (protractory in Theria). The Taeniolabididae differ from the Eucosmodontidae in having a more powerful masticatory musculature, expressed by the higher zygomatic arch with relatively larger anterior and middle zygomatic ridges and higher coronoid process. It is speculated that the bicuspid, or pointed upper incisors, and semi-procumbent, pointed lower ones, characteristic of non- taeniolabidoid mdtitliberculates were used for picking-up and killing insects or other prey. In relation to the backward power stroke the low position of the condylar process was advantageous for most multituberculates. In extreme cases (Sloanbaataridae and Taeniolabididae), the adaptation for crushing hard seeds, worked against the benefit of the low position of the condylar process and a high condylar process developed. Five new multituberculate autapomorphies are rec- ognized: anterior and intermediate zygomatic ridges: glenoid fossa large, flat and sloping backwards (forwards in rodents), arranged anterolateral and standing out from the braincase; semicircular posterior margin of the dentary with condylar process forming at least a part of it; anterior position of the coronoid process; and anterior position of the masseteric fossa. -

New Specimens of the Multituberculate Mammal Sphenopsalis from China: Implications for Phylogeny and Biology of Taeniolabidoids
New specimens of the multituberculate mammal Sphenopsalis from China: Implications for phylogeny and biology of taeniolabidoids FANG-YUAN MAO, YUAN-QING WANG, and JIN MENG Mao, F.-Y., Wang, Y.-Q., and Meng, J. 2016. New specimens of the multituberculate mammal Sphenopsalis from China: Implications for phylogeny and biology of taeniolabidoids. Acta Palaeontologica Polonica 61 (2): 429–454. Multituberculates are the most diverse and best known group of Mesozoic mammals; they also persisted into the Paleogene and became extinct in the Eocene, possibly outcompeted by rodents that have similar morphological and pre- sumably ecological adaptations. Among the Paleogene multituberculates, those that have the largest body sizes belong to taeniolabidoids, which contain several derived species from North America and Asia and some species with uncertain taxonomic positions. Of the known taeniolabidoids, the poorest known taxon is Sphenopsalis nobilis from Mongolia and Inner Mongolia, China, represented previously by a few isolated teeth. Its relationship with other multituberculates thus has remained unclear. Here we report new specimens of Sphenopsalis nobilis collected from the upper Paleocene of the Erlian Basin, Inner Mongolia, China, during a multi-year field effort beginning in 2000. These new specimens document substantial parts of the dental, partial cranial and postcranial morphologies of Sphenopsalis, including the upper and lower incisors, partial premolars, complete upper and lower molars, a partial rostrum, fragments of the skull roof, middle ear cavity, a partial scapula, and partial limb bones. With the new specimens we are able to present a detailed description of Sphenopsalis, comparisons among relevant taeniolabidoids, and brief phylogenetic analyses based on a dataset consisting of 43 taxa and 102 characters.