Matsucoccus Macrocicatrices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Biogeography of an Emergent Forest Pest, Matsucoccus Macrocicatrices
Received: 19 April 2019 | Revised: 7 August 2019 | Accepted: 12 August 2019 DOI: 10.1111/jbi.13702 RESEARCH PAPER Native or non‐native? Historical biogeography of an emergent forest pest, Matsucoccus macrocicatrices Thomas D. Whitney1,2 | Kamal J. K. Gandhi1 | Rima D. Lucardi2 1Puyallup Research and Extension Center, Washington State University, Abstract Puyallup, WA 98371, USA Aim: A historically benign insect herbivore, Matsucoccus macrocicatrices, has re‐ 2 USDA Forest Service, Southern Research cently been linked to dieback and mortality of eastern white pine (Pinus strobus L.). Station, 320 E. Green Street, Athens, GA 30602, USA Previous reports indicated that its native range was restricted to New England, USA and southeastern Canada. Now, the insect occurs throughout an area extending from Correspondence Thomas D. Whitney, Puyallup Research the putative native range, southward to Georgia, and westward to Wisconsin. Our and Extension Center, Washington State goal was to evaluate whether its current distribution was due to recent introduc‐ University, Puyallup, WA 98371, USA. Email: [email protected] tions consistent with invasion processes. We considered two hypotheses: (a) if recent expansion into adventive regions occurred, those populations would have reduced Funding information USDA Forest Service, Southern genetic diversity due to founder effect(s); alternatively (b) if M. macrocicatrices is na‐ Research Station, Grant/Award tive and historically co‐occurred with its host tree throughout the North American Number: 13‐CA‐11330129‐056 and 16‐ CS‐11330129‐045; Southern Region (8)‐ range, then populations would have greater overall genetic diversity and a population Forest Health Protection; USDA Agricultural structure indicative of past biogeographical influences. -

Zootaxa,Phylogeny and Higher Classification of the Scale Insects
Zootaxa 1668: 413–425 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea)* P.J. GULLAN1 AND L.G. COOK2 1Department of Entomology, University of California, One Shields Avenue, Davis, CA 95616, U.S.A. E-mail: [email protected] 2School of Integrative Biology, The University of Queensland, Brisbane, Queensland 4072, Australia. Email: [email protected] *In: Zhang, Z.-Q. & Shear, W.A. (Eds) (2007) Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa, 1668, 1–766. Table of contents Abstract . .413 Introduction . .413 A review of archaeococcoid classification and relationships . 416 A review of neococcoid classification and relationships . .420 Future directions . .421 Acknowledgements . .422 References . .422 Abstract The superfamily Coccoidea contains nearly 8000 species of plant-feeding hemipterans comprising up to 32 families divided traditionally into two informal groups, the archaeococcoids and the neococcoids. The neococcoids form a mono- phyletic group supported by both morphological and genetic data. In contrast, the monophyly of the archaeococcoids is uncertain and the higher level ranks within it have been controversial, particularly since the late Professor Jan Koteja introduced his multi-family classification for scale insects in 1974. Recent phylogenetic studies using molecular and morphological data support the recognition of up to 15 extant families of archaeococcoids, including 11 families for the former Margarodidae sensu lato, vindicating Koteja’s views. Archaeococcoids are represented better in the fossil record than neococcoids, and have an adequate record through the Tertiary and Cretaceous but almost no putative coccoid fos- sils are known from earlier. -

Scientific Name Species Common Name Abies Lasiocarpa FIR Subalpine Acacia Macracantha ACACIA Long-Spine
Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek Alnus iridis ssp. sinuata ALDER Sitka Alnus maritima ALDER Seaside Alvaradoa amorphoides ALVARADOA Mexican Amelanchier laevis SERVICEBERRY Allegheny Amyris balsamifera TORCHWOOD Balsam Annona squamosa SUGAR-APPLE NA Araucaria cunninghamii ARAUCARIA Cunningham Arctostaphylos glauca MANZANITA Bigberry Asimina obovata PAWPAW Bigflower Bourreria radula STRONGBACK Rough Brasiliopuntia brasiliensis PRICKLY-PEAR Brazilian Bursera simaruba GUMBO-LIMBO NA Caesalpinia pulcherrima FLOWERFENCE NA Capparis flexuosa CAPERTREE Limber CRUCIFIXION- Castela emoryi THORN NA Casuarina equisetifolia CASUARINA Horsetail Ceanothus arboreus CEANOTHUS Feltleaf Ceanothus spinosus CEANOTHUS Greenbark Celtis lindheimeri HACKBERRY Lindheimer Celtis occidentalis HACKBERRY Common Cephalanthus occidentalis BUTTONBUSH Common Cercis canadensis REDBUD Eastern Cercocarpus traskiae CERCOCARPUS Catalina Chrysophyllum oliviforme SATINLEAF NA Citharexylum berlandieri FIDDLEWOOD Berlandier Citrus aurantifolia LIME NA Citrus sinensis ORANGE Orange Coccoloba uvifera SEAGRAPE NA Colubrina arborescens COLUBRINA Coffee Colubrina cubensis COLUBRINA Cuba Condalia globosa -

The <I>Matsucoccus</I> Cockerell, 1909 of Florida (Hemiptera
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 9-30-2020 The Matsucoccus Cockerell, 1909 of Florida (Hemiptera: Coccomorpha: Matsucoccidae): Potential pests of Florida pines Muhammad Z. Ahmed Charles H. Ray Matthew R. Moore Douglass R. Miller Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Ecology and Evolutionary Biology Commons, and the Entomology Commons This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. InsectaA journal of world insect systematics Mundi 0810 The Matsucoccus Cockerell, 1909 of Florida Page Count: 31 (Hemiptera: Coccomorpha: Matsucoccidae): Potential pests of Florida pines Muhammad Z. Ahmed Florida State Collection of Arthropods Division of Plant Industry, Florida Department of Agriculture and Consumer Services 1911 SW 34th Street Gainesville, FL 32608, USA [email protected] Charles H. Ray Department of Entomology and Plant Pathology Auburn University Museum of Natural History Room 301, Funchess Hall Auburn University, AL 36849, USA Matthew R. Moore Molecular Diagnostics Laboratory Division of Plant Industry, Florida Department of Agriculture and Consumer Services 1911 SW 34th Street Gainesville, FL 32608, USA Douglass R. Miller Florida State Collection of Arthropods Division of Plant Industry, Florida Department of Agriculture and Consumer Services 1911 SW 34th Street Gainesville, FL 32608, USA Date of issue: October 30, 2020 Center for Systematic Entomology, Inc., Gainesville, FL Ahmed MZ, Ray CH, Moore MR, Miller DR. -

Dry White Pine (Hemlock) Oak Forest
Dry white pine (hemlock) oak forest This community type occurs on fairly dry sites, often with 25% or more of the forest floor covered by rocks, boulders and/or exposed bedrock. The canopy may be somewhat open and tree growth somewhat suppressed. The tree stratum is dominated by a mixture of Pinus strobus (eastern white pine), or occasionally Tsuga canadensis (eastern hemlock), and a mixture of dry-site hardwoods, predominantly oaks. On most sites, the conifer and the hardwood component both range between 25% and 75% of the canopy. The oak species most often associated with this type are Quercus montana (chestnut oak), and Q. alba (white oak), although Q. velutina (black oak), Q. coccinea (scarlet oak), or Q. rubra (northern red oak) may also occur. Other associated trees include Nyssa sylvatica (black-gum), Betula lenta (sweet birch), Fraxinus americana (white ash), Prunus serotina (wild black cherry), and Castanea dentata (American chestnut) sprouts. There is often a heath-dominated shrub layer with Kalmia latifolia (mountain laurel) being especially important; Gaylussacia baccata (black huckleberry), Vaccinium spp. (blueberries), and Kalmia angustifolia (sheep laurel) are also common. Other shrubs, like Cornus florida (flowering dogwood), Hamamelis virginiana (witch-hazel), Viburnum acerifolium (maple-leaved viburnum) may also occur on less acidic sites. There is typically a sparse herbaceous layer with a northern affinity; Aralia nudicaulis (wild sarsaparilla), Pteridium aquilinum (bracken fern), Maianthemum canadense (Canada mayflower), Gaultheria procumbens (teaberry), Trientalis borealis (star-flower), and Medeola virginiana (Indian cumber-root) are typical. The successional status of this type seems variable, in some cases, especially on harsher sites, it appears relatively stable, in other cases it appears to be transitional. -

Bacterial Associates of Orthezia Urticae, Matsucoccus Pini, And
Protoplasma https://doi.org/10.1007/s00709-019-01377-z ORIGINAL ARTICLE Bacterial associates of Orthezia urticae, Matsucoccus pini, and Steingelia gorodetskia - scale insects of archaeoccoid families Ortheziidae, Matsucoccidae, and Steingeliidae (Hemiptera, Coccomorpha) Katarzyna Michalik1 & Teresa Szklarzewicz1 & Małgorzata Kalandyk-Kołodziejczyk2 & Anna Michalik1 Received: 1 February 2019 /Accepted: 2 April 2019 # The Author(s) 2019 Abstract The biological nature, ultrastructure, distribution, and mode of transmission between generations of the microorganisms associ- ated with three species (Orthezia urticae, Matsucoccus pini, Steingelia gorodetskia) of primitive families (archaeococcoids = Orthezioidea) of scale insects were investigated by means of microscopic and molecular methods. In all the specimens of Orthezia urticae and Matsucoccus pini examined, bacteria Wolbachia were identified. In some examined specimens of O. urticae,apartfromWolbachia,bacteriaSodalis were detected. In Steingelia gorodetskia, the bacteria of the genus Sphingomonas were found. In contrast to most plant sap-sucking hemipterans, the bacterial associates of O. urticae, M. pini, and S. gorodetskia are not harbored in specialized bacteriocytes, but are dispersed in the cells of different organs. Ultrastructural observations have shown that bacteria Wolbachia in O. urticae and M. pini, Sodalis in O. urticae, and Sphingomonas in S. gorodetskia are transovarially transmitted from mother to progeny. Keywords Symbiotic microorganisms . Sphingomonas . Sodalis-like -

American Museum Novitates
AMERICAN MUSEUM NOVITATES Number 3823, 80 pp. January 16, 2015 Diverse new scale insects (Hemiptera: Coccoidea) in amber from the Cretaceous and Eocene with a phylogenetic framework for fossil Coccoidea ISABELLE M. VEA1, 2 AND DAVID A. GRIMALDI2 ABSTRACT Coccoids are abundant and diverse in most amber deposits around the world, but largely as macropterous males. Based on a study of male coccoids in Lebanese amber (Early Cretaceous), Burmese amber (Albian-Cenomanian), Cambay amber from western India (Early Eocene), and Baltic amber (mid-Eocene), 16 new species, 11 new genera, and three new families are added to the coccoid fossil record: Apticoccidae, n. fam., based on Apticoccus Koteja and Azar, and includ- ing two new species A. fortis, n. sp., and A. longitenuis, n. sp.; the monotypic family Hodgsonicoc- cidae, n. fam., including Hodgsonicoccus patefactus, n. gen., n. sp.; Kozariidae, n. fam., including Kozarius achronus, n. gen., n. sp., and K. perpetuus, n. sp.; the irst occurrence of a Coccidae in Burmese amber, Rosahendersonia prisca, n. gen., n. sp.; the irst fossil record of a Margarodidae sensu stricto, Heteromargarodes hukamsinghi, n. sp.; a peculiar Diaspididae in Indian amber, Nor- markicoccus cambayae, n. gen., n. sp.; a Pityococcidae from Baltic amber, Pityococcus monilifor- malis, n. sp., two Pseudococcidae in Lebanese and Burmese ambers, Williamsicoccus megalops, n. gen., n. sp., and Gilderius eukrinops, n. gen., n. sp.; an Early Cretaceous Weitschatidae, Pseudo- weitschatus audebertis, n. gen., n. sp.; four genera considered incertae sedis, Alacrena peculiaris, n. gen., n. sp., Magnilens glaesaria, n. gen., n. sp., and Pedicellicoccus marginatus, n. gen., n. sp., and Xiphos vani, n. -

Coccidology. the Study of Scale Insects (Hemiptera: Sternorrhyncha: Coccoidea)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ciencia y Tecnología Agropecuaria (E-Journal) Revista Corpoica – Ciencia y Tecnología Agropecuaria (2008) 9(2), 55-61 RevIEW ARTICLE Coccidology. The study of scale insects (Hemiptera: Takumasa Kondo1, Penny J. Gullan2, Douglas J. Williams3 Sternorrhyncha: Coccoidea) Coccidología. El estudio de insectos ABSTRACT escama (Hemiptera: Sternorrhyncha: A brief introduction to the science of coccidology, and a synopsis of the history, Coccoidea) advances and challenges in this field of study are discussed. The changes in coccidology since the publication of the Systema Naturae by Carolus Linnaeus 250 years ago are RESUMEN Se presenta una breve introducción a la briefly reviewed. The economic importance, the phylogenetic relationships and the ciencia de la coccidología y se discute una application of DNA barcoding to scale insect identification are also considered in the sinopsis de la historia, avances y desafíos de discussion section. este campo de estudio. Se hace una breve revisión de los cambios de la coccidología Keywords: Scale, insects, coccidae, DNA, history. desde la publicación de Systema Naturae por Carolus Linnaeus hace 250 años. También se discuten la importancia económica, las INTRODUCTION Sternorrhyncha (Gullan & Martin, 2003). relaciones filogenéticas y la aplicación de These insects are usually less than 5 mm códigos de barras del ADN en la identificación occidology is the branch of in length. Their taxonomy is based mainly de insectos escama. C entomology that deals with the study of on the microscopic cuticular features of hemipterous insects of the superfamily Palabras clave: insectos, escama, coccidae, the adult female. -

Specializing in Rare and Unique Trees 2015 Catalogue
Whistling Gardens 698 Concession 3, Wilsonville, ON N0E 1Z0 Phone- 519-443-5773 Fax- 519-443-4141 Specializing in Rare and Unique Trees 2015 Catalogue Pot sizes: The number represents the size of the pot ie. #1= 1 gallon, #10 = 10 gallon #1 potted conifers are usually 3-5years old. #10 potted conifers dwarf conifers are between 10 and 15 years old #1 trees= usually seedlings #10 trees= can be several years old anywhere from 5 to 10' tall depending on species and variety. Please ask us on sizes and varieties you are not sure about. Many plants are limited to 1specimen. To reserve your plant(s) a 25% is required. Plants should be picked up by June 15th. Most plants arrive at the gardens by May 10th. Guarantee: We cannot control the weather (good or bad), rodents (big or small), pests (teenie, tiny), poor siting, soil types, lawnmovers, snowplows etc. Plants we carry are expected to grow within the parameters of normal weather conditons. All woody plant purchases are guaranteed from time of purchase to December 1st of current year Any plant not performing or dying in current season will be happily replaced or credited towards a new plant. Please email us if possible with any info needed about our plants. We do not have a phone in the garden centre and I'm rarely in the office. It is very helpful to copy and paste the botanical name of the plant into your Google browser, in most cases, a d Order Item Size Price Description of Pot European White Fir Zone 5 Abies alba Barabit's Spreader 25-30cm #3 $100.00 Abies alba Barabit's Spreader 40cm -

Deer-Resistant Landscape Plants This List of Deer-Resistant Landscape Plants Was Compiled from a Variety of Sources
Deer-Resistant Landscape Plants This list of deer-resistant landscape plants was compiled from a variety of sources. The definition of a deer-resistant plant is one that may be occasionally browsed, but not devoured. If deer are hungry enough or there’s a limited amount of food available, they will eat almost anything. Perennials, Herbs & Bulbs Achillea Yarrow Lavandula augustifolia Lavender Adiatum pedatum Maiden Hair Fern Liatris Gayfeather Agastache cana Agastache Lychnis chalcedonica Maltese Cross Ajuga reptans Bugleweed Matteuccia Ostrich Fern Alchemilla Lady’s Mantle Mentha Mint Allium spp. Flowering Onion Miscanthus Silver Grass Arabis Rock Cress Myosotis sylvatica Forget-Me-Not Armeria maritime Sea Thrift Narcissus Daffodils & Narcissus Artemisia Wormwood Nepeta Catmint Asarum europaeum Wild Ginger Oenothera Evening Primrose Asclepias tuberosa Butterfly Weed Paeonia Peony Bergenia Bergenia Papaver orientale Oriental Poppy Calamagrostis Reed Grass Pennisetum orientale Oriental Fountain Grass Cerastium Snow-In-Summer Perovskia Russian Sage Cimcifuga Bugbane Physostegia Obedient Plant Convallaria Lily-of-the-Valley Platycodon Balloon Flower Corydalis lutea Gold Bleeding Heart Polygonatum Solomon’s Seal Dicentra Bleeding Heart Pulmonaria Lungwort Digitalis Foxglove Rudbeckia Black Eyed Susan Echinops Globe Thistle Saponaria Soapwort Festuca ovinia Blue Fescue Salvia Sage Galanthus nivalis Snowdrop Sedum Stonecrop Helleborus Hellebores Solidago Goldenrod Iris sibirica & ensata Japanese & Siberian Iris Stachys byzantina Lamb’s Ear Lamium -
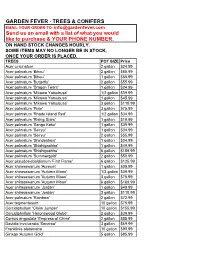
Trees & Conifers
GARDEN FEVER - TREES & CONIFERS EMAIL YOUR ORDER TO: [email protected] Send us an email with a list of what you would like to purchase & YOUR PHONE NUMBER. ON HAND STOCK CHANGES HOURLY. SOME ITEMS MAY NO LONGER BE IN STOCK, ONCE YOUR ORDER IS PLACED. TREES POT SIZE Price Acer ciricinatum 2 gallon $24.99 Acer palmatum 'Bihou' 2 gallon $85.99 Acer palmatum 'Bihou' 1 gallon $55.99 Acer palmatum 'Butterfly' 2 gallon $55.99 Acer palmatum 'Dragon Tears' 1 gallon $24.99 Acer palmatum 'Mikawa Yatsubusa' 1/2 gallon $39.99 Acer palmatum 'Mikawa Yatsubusa' 1 gallon $45.99 Acer palmatum 'Mikawa Yatsubusa' 3 gallon $110.99 Acer palmatum 'Pixie' 3 gallon $75.99 Acer palmatum 'Rhode Island Red' 1/2 gallon $34.99 Acer palmatum 'Rising Stars' 1 gallon $19.99 Acer palmatum 'Sango Kaku' 1 gallon $39.99 Acer palmatum 'Seiryu' 1 gallon $34.99 Acer palmatum 'Seiryu' 2 gallon $55.99 Acer palmatum 'Shindeshojo' 1 gallon $34.99 Acer palmatum 'Shishigashira' 1 gallon $49.99 Acer palmatum 'Shishigashira' 6 gallon $159.99 Acer palmatum 'Summergold' 2 gallon $59.99 Acer pseudosieboldianum 'First Flame' 6 gallon $125.99 Acer shirasawanum 'Aureum' 1 gallon $39.99 Acer shirasawanum 'Autumn Moon' 1/2 gallon $39.99 Acer shirasawanum 'Autumn Moon' 3 gallon $75.99 Acer shirasawanum 'Autumn Moon' 6 gallon $169.99 Acer shirasawanum 'Jordan' 1 gallon $49.99 Acer shirasawanum 'Jordan' 3 gallon $110.99 Acer palmatum 'Rainbow' 2 gallon $72.99 Acer tegmentosum 10 gallon $75.99 Cercidiphyllum 'Claim Jumper' 10 gallon $155.99 Cercidiphyllum 'Heronswood Globe' 2 gallon $39.99 Cornus -

Nursery Catalog
Tel: 503.628.8685 Fax: 503.628.1426 www.eshraghinursery.com 1 Eshraghi’s TOP 10 picks Our locations 1 Main Office, Shipping & Growing 2 Retail Store & Growing 26985 SW Farmington Road Farmington Gardens Hillsboro, OR 97123 21815 SW Farmington Road Beaverton, OR 97007 1 2 3 7 6 3 River Ranch Facility 4 Liberty Farm 4 5 10 N SUNSET HWY TO PORTLAND 8 9 TU HILLSBORO ALA TIN 26 VALL SW 185TH AVE. EY HWY. #4 8 BEAVERTON TONGUE LN. GRABEL RD . D R . E D G R ID E ALOHA R G B D I R R #3 SW 209TH E B T D FARMINGTON ROAD D N A I SIMPSON O O M O R R 10 217 ROSEDALE W R E S W V S I R N W O 219 T K C A J #2 #1 SW UNGER RD. SW 185TH AVE. 1 Acer circinatum ‘Pacific Fire’ (Vine Maple), page 6 D A SW MURRAY BLVD. N RO 2 palmatum (Japanese Maple), NGTO Acer 'Geisha Gone Wild' page 8 FARMI 3 Acer palmatum 'Mikawa yatsubusa' (Japanese Maple), page 10 #1 4 Acer palmatum dissectum 'Orangeola' (Japanese Maple), page 14 5 Hydrangea macrophylla 'McKay', Cherry Explosion PP28757 (Hydrangea), page 32 6 Picea glauca 'Eshraghi1', Poco Verde (White Spruce), page 61 ROAD HILL CLARK 7 Picea pungens 'Hockersmith', Linda (Colorado Spruce), page 64 RY ROAD 8 Pinus nigra 'Green Tower' (Austrian Pine), page 65 SCHOLLS FER 9 Thuja occidentalis 'Janed Gold', Highlights™ PP21967 (Arborvitae), page 70 10 Thuja occidentalis 'Anniek', Sienna Sunset™ (Arborvitae), page 69 Table of contents Tags Make a Difference .