Monsanto Petition (19-091-01P) for Determination of Nonregulated Status for Insect-Protected MON 88702 Cotton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Impact of the Nation's Most Widely Used Insecticides on Birds
The Impact of the Nation’s Most Widely Used Insecticides on Birds Neonicotinoid Insecticides and Birds The Impact of the Nation’s Most Widely Used Insecticides on Birds American Bird Conservancy, March 2013 Grasshopper Sparrow by Luke Seitz Cover photos: Horned Lark and chicks by Middleton Evans; Corn field, stock.xchng, sxc.hu; Calico Pennant dragonfly by David Cappaert, Michigan State University, Bugwood.org 1 Neonicotinoid Insecticides and Birds American Bird Conservancy would like to thank the Turner Foundation, Wallace Genetic Foundation, Jeff and Connie Woodman, Cornell Douglas Foundation and A.W. Berry Foundation for their ongoing support for American Bird Conservancy’s Pesticides Program. Written by Dr. Pierre Mineau and Cynthia Palmer Designed by Stephanie von Blackwood About the Authors Dr. Pierre Mineau began his long and distinguished scientific career studying the effects of persistent organochlorine compounds, like DDT and PCBs, on fish-eating birds. He then became responsible for the Canadian assessment of new and existing pesticides to determine their adverse impacts on wildlife. In 1994 he transitioned from regulatory reviews to full-time research on the environmental impacts of pesticides, achieving the rank of Senior Research Scientist at Environment Canada. Working with international collaborators and graduate students, he works on assessing globally the environmental footprint of pesticides. He also studies how birds are exposed to pesticides and how bird populations respond to pesticide use and agricultural practices. His work includes defining the ecological values of birds in cropland as well as estimating the incidental take of birds from various other human activities. He has written more than 100 peer-reviewed publications and has authored some 200 presentations. -

Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects
EN58CH06-Casida ARI 5 December 2012 8:11 Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects John E. Casida1,∗ and Kathleen A. Durkin2 1Environmental Chemistry and Toxicology Laboratory, Department of Environmental Science, Policy, and Management, 2Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, California 94720; email: [email protected], [email protected] Annu. Rev. Entomol. 2013. 58:99–117 Keywords The Annual Review of Entomology is online at acetylcholinesterase, calcium channels, GABAA receptor, nicotinic ento.annualreviews.org receptor, secondary targets, sodium channel This article’s doi: 10.1146/annurev-ento-120811-153645 Abstract Copyright c 2013 by Annual Reviews. Neuroactive insecticides are the principal means of protecting crops, people, All rights reserved livestock, and pets from pest insect attack and disease transmission. Cur- ∗ Corresponding author rently, the four major nerve targets are acetylcholinesterase for organophos- phates and methylcarbamates, the nicotinic acetylcholine receptor for neonicotinoids, the γ-aminobutyric acid receptor/chloride channel for by Public Health Information Access Project on 04/29/14. For personal use only. Annu. Rev. Entomol. 2013.58:99-117. Downloaded from www.annualreviews.org polychlorocyclohexanes and fiproles, and the voltage-gated sodium channel for pyrethroids and dichlorodiphenyltrichloroethane. Species selectivity and acquired resistance are attributable in part to structural differences in binding subsites, receptor subunit interfaces, or transmembrane regions. Additional targets are sites in the sodium channel (indoxacarb and metaflumizone), the glutamate-gated chloride channel (avermectins), the octopamine receptor (amitraz metabolite), and the calcium-activated calcium channel (diamides). Secondary toxic effects in mammals from off-target serine hydrolase inhibi- tion include organophosphate-induced delayed neuropathy and disruption of the cannabinoid system. -

Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template For
water Article Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template for Food Safety Assessment Ayodeji O. Adegun 1, Thompson A. Akinnifesi 1, Isaac A. Ololade 1 , Rosa Busquets 2 , Peter S. Hooda 3 , Philip C.W. Cheung 4, Adeniyi K. Aseperi 2 and James Barker 2,* 1 Department of Chemical Sciences, Adekunle Ajasin University, Akungba Akoko P.M.B. 001, Ondo State, Nigeria; [email protected] (A.O.A.); [email protected] (T.A.A.); [email protected] (I.A.O.) 2 School of Life Sciences, Pharmacy and Chemistry, Kingston University, Kingston-upon-Thames KT1 2EE, UK; [email protected] (R.B.); [email protected] (A.K.A.) 3 School of Engineering and the Environment, Kingston University, Kingston-on-Thames KT1 2EE, UK; [email protected] 4 Department of Chemical Engineering, Imperial College, London SW7 2AZ, UK; [email protected] * Correspondence: [email protected] Received: 17 June 2020; Accepted: 24 August 2020; Published: 28 August 2020 Abstract: The Owena River Basin in Nigeria is an area of agricultural importance for the production of cocoa. To optimise crop yield, the cocoa trees require spraying with neonicotinoid insecticides (Imidacloprid, Thiacloprid Acetamiprid and Thiamethoxam). It is proposed that rainwater runoff from the treated area may pollute the Owena River and that these pesticides may thereby enter the human food chain via six species of fish (Clarias gariepinus, Clarias anguillaris, Sarotherodon galilaeus, Parachanna obscura, Oreochromis niloticus and Gymnarchus niloticus) which are cultured in the river mostly for local consumption. -
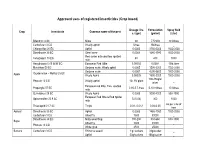
Approved Uses of Registered Insecticides (Crop Based)
Approved uses of registered insecticides (Crop based) Dosage / ha Formulation Spray fluid Crop Insecticide Common name of the pest a.i (gm) (gm/ml) (Liter) Bifenthrin 8 SC Mites 60 7.5ml/lit 10 lit/tree Carbofuran 3 CG Woolly aphid 5/tree 166/tree _ Chlorpyrifos 20 EC Aphid 0.0005 3750-5000 1500-2000 Dimethoate 30 EC Stem borer 0.0003 1485-1980 1500-2000 Red spider mite and two spotted Fenazaquin 10 EC 40 400 1000 mite Hexythiazox 5.45 W/W EC European Red Mite 0.00002 0.0004 10ltr./tree Malathion 50 EC Sanjose scale, Wooly aphid 0.0005 1500-2000 1500-2000 Sanjose scale 0.0007 4200-5600 1500-2000 Oxydemeton – Methyl 25 EC Apple Wooly Aphid 0.00025 1500-2000 1500-2000 100-150gm/ Phorate 10 CG Woolly aphid 10- 15/ plant _ plant European red Mite, Two spotted Propargite 57 EC 2.85-5.7 /tree 5-10 ml/tree 10 lit/tree mite Quinalphos 25 EC Wooly Aphid 0.0005 3000-4000 500-1000 European Red Mite & Red Spider Spiromesifen 22.9 SC 72(0.03) 300 1000 mite As per size of Thiacloprid 21.7 SC Thrips 0.01- 0.012 0.04-0.05 tree Apricot Dimethoate 30 EC Aphid 0.0003 1485-1980 1500-2000 Carbofuran 3 CG Shoot fly 1500 50000 _ Dimethoate 30 EC Milky weed bug 180-200 594-660 500-1000 Bajra Shoot fly 3000 30000 _ Phorate 10 CG White grub 2500 25000 _ Banana Carbofuran 3 CG Rhizome weevil 1 g/ suckers 33g/sucker _ Aphid 50g/suckers 166g/sucker _ Nematode 1.5g/suckers 50g/suckers _ Dimethoate 30 EC Aphid, Lace wing bug 0.0003 1485-1980 1500-2000 Tingyi bug 0.00025 1500-2000 1500-2000 Oxydemeton – Methyl 25 EC Aphids 0.0005 3000-4000 1500-2000 2.5 -1.25/ 25 -12.5/ Phorate 10 CG Aphid _ plant plant Quinalphos 25 EC Tingid bug 0.0005 3000-4000 500-1000 Phosalone 35 EC Aphid 500 1428 500-1000 Aphid 1000 33300 _ Barely Carbofuran 3 CG Jassid 1250 41600 _ Cyst nematode 1000 33300 _ Phorate 10 CG Aphid 1000 10000 _ Beans Chlorpyrifos 20 EC Pod borer , Black bug 600 3000 500-1000 Chlorantraniliprole 18.5 SC Pod borers 25 125 500 Chlorpyrifos 1.5 DP Helicoverpa armigera 375 25000 _ Azadirachtin 0.03 (300 PPM) Pod Borer _ _ _ Bacillus thuringiensis Var. -

Cyantraniliprole
PUBLIC RELEASE SUMMARY on the Evaluation of the New Active Constituent Cyantraniliprole in the Product DuPont Exirel Insecticide APVMA Product Number 64103 OCTOBER 2013 © Australian Pesticides and Veterinary Medicines Authority 2013 ISSN: 1443-1335 (electronic) ISBN: 978-1-922188-50-2 (electronic) Ownership of intellectual property rights in this publication Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Australian Pesticides and Veterinary Medicines Authority (APVMA). Creative Commons licence With the exception of the Coat of Arms and other elements specifically identified, this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence. This is a standard form agreement that allows you to copy, distribute, transmit and adapt this publication provided that you attribute the work. A summary of the licence terms is available from www.creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from www.creativecommons.org/licenses/by/3.0/au/legalcode. The APVMA’s preference is that you attribute this publication (and any approved material sourced from it) using the following wording: Source: licensed from the Australian Pesticides and Veterinary Medicines Authority (APVMA) under a Creative Commons Attribution 3.0 Australia Licence. In referencing this document the Australian Pesticides and Veterinary Medicines Authority should be cited as author, publisher and copyright owner. Use of the Coat of Arms The terms under which the Coat of Arms can be used are set out on the Department of the Prime Minister and Cabinet website (see www.dpmc.gov.au/guidelines). -

Reducing Reliance on Insecticides to Manage Spotted Wing Drosophila
Reducing reliance on insecticides to manage spotted wing drosophila Hannah Burrack, Katie Swoboda-Bhattarai, and Lauren Diepenbrock Department of Entomology Topics New SWD initiatives Research updates Pesticide rotation programs Timing insecticide applications Recommendations Development and Implementation of Systems-Based Organic Management Strategies for Spotted Wing Drosophila Three years: 1 Sept 2015 through 31 Aug 2018 Project goals: Support the development of novel organic SWD management strategies that will increase NOP compliant management options for growers and decrease their reliance on broad-spectrum insecticides. USDA National Institute for Food and Agriculture (NIFA) Organic Agriculture Research and Extension Initiative Award Number 2015-51300-24154 OREI Objectives Objective 1: Develop semiochemical-based behavioral management tactics for SWD Objective 2: Develop cultural control tactics and evaluate their efficacy and feasibility for reduction of SWD damage Objective 3: Develop effective chemical control strategies that minimally disrupt biological suppression of pest complexes Objective 4: Develop an integrated outreach approach to evaluate and implement organic SWD management strategies Project Director: Ash Sial Ahmad University of Georgia Co-Project Directors: Hannah Burrack North Carolina State University Matt Grieshop Michigan State University Christelle Guedot University of Wisconsin-Madison Kelly Hamby University of Maryland Rufus Isaacs Michigan State University Donn Johnson University of Arkansas Jana Lee United -

Toxicological Studies on Boric Acid, Imidacloprid And
TOXICOLOGICAL STUDIES ON BORIC ACID, IMIDACLOPRID AND FIPRONIL AND THEIR BINARY MIXTURES AS INSECTICIDES ON GERMAN COCKROACH Blattellagermanica (L.) (DICTYOPTERA: BLATTELLIDAE) By FATMA SHERIF AHMED B.Sc. Agric. Sci. (Pesticides), Fac. Agric., Cairo Univ., 2007 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE In Agricultural Sciences (Pesticides) Department of Economic Entomology and Pesticides Faculty of Agriculture Cairo University EGYPT 2015 ١ INTRODUCTION The German cockroaches, Blattellagermanica (L.), (Dictyoptera, Blattellidae) are the most common indoor pests, especially in multiple-family housing and the most significant pest in many parts of the world (Goddard, 2003). German cockroaches prefer warm, wet locations with high humidity such as kitchens, bathrooms and laundry areas. These conditions are available in several places as homes, apartments, restaurants, supermarkets, hospitals and other buildings where food are stored. Cockroaches are not only corrupt food but also transfer pathogens such as Salmonella, Shigella, Escherichia coli, Staphylococcus aureus and Bacillus cereus (Baumholtz et al., 1997 and Tachbeleet al., 2006). Medically important parasites such as bacteria, fungi and molds, protozoans, viruses were isolated from external and internal surface of cockroach (Brenner, 1995). Cockroaches can also transfer both gram-positive and negative bacteria (El-Sherbini and El- Sherbini, 2011). A large number of neurotoxic and non-neurotoxic insecticides were used for German cockroach control, as this pest has a considerable ability to develop resistance to a variety of chemical insecticides(Cochran, 1989 and 1995a; Scott et al., 1990; Rust and Reierson, 1991; Rust et al., 1993; Holbrook et al., 1999; Espinosa-Islas et al., 2002 and Rahayuet al., 2012). -

Proposed Interim Registration Review Decision for Imidacloprid
Docket Number EPA-HQ-OPP-2008-0844 www.regulations.gov Imidacloprid Proposed Interim Registration Review Decision Case Number 7605 January 2020 Approved by: Elissa Reaves, Ph.D. Acting Director Pesticide Re-evaluation Division Date: __ 1-22-2020 __ Docket Number EPA-HQ-OPP-2008-0844 www.regulations.gov Table of Contents I. INTRODUCTION .................................................................................................................. 4 A. Summary of Imidacloprid Registration Review............................................................... 5 B. Summary of Public Comments on the Draft Risk Assessments and Agency Responses 7 II. USE AND USAGE ............................................................................................................... 14 III. SCIENTIFIC ASSESSMENTS ......................................................................................... 15 A. Human Health Risks....................................................................................................... 15 1. Risk Summary and Characterization .......................................................................... 15 2. Human Incidents and Epidemiology .......................................................................... 17 3. Tolerances ................................................................................................................... 18 4. Human Health Data Needs ......................................................................................... 18 B. Ecological Risks ............................................................................................................ -

CYANTRANILIPROLE (263) the First Draft Was Prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand
Cyantraniliprole 361 CYANTRANILIPROLE (263) The first draft was prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand EXPLANATION Cyantraniliprole is a diamide insecticide with a mode of action (ryanodine receptor activation) similar to chlorantraniliprole and flubendiamide. It has root systemic activity with some translaminar movement and is effective against the larval stages of lepidopteran insects; and also on thrips, aphids, and some other chewing and sucking insects. Authorisations exist for the use of cyantraniliprole in Canada, Columbia, Malaysia, New Zealand, Vietnam and the CLISS countries in West Africa. Authorisations are also being progressed in Australia, Europe and USA under an OECD Joint Review exercise. Cyantraniliprole was scheduled by the Forty-fourth Session of the CCPR as a new compound for consideration by the 2013 JMPR. Residue and analytical aspects of cyantraniliprole were considered for the first time by the present meeting. The manufacturer submitted studies on metabolism, analytical methods, supervised field trials, processing, freezer storage stability, environmental fate in soil and rotational crop residues. In this evaluation, the values presented in the tables are as reported in the various studies, but in the accompanying text, they have generally been rounded to two significant digits. Abbreviations have also been used for the various cyantraniliprole metabolites mentioned in the study reports. These include: IN-F6L99 3-Bromo-N-methyl-1H-pyrazole-5-carboxamide IN-HGW87 -

Effects of Cyantraniliprole, a Novel Anthranilic Diamide Insecticide, Against Asian Citrus Psyllid Under Laboratory and Field Co
Submitted to: Correspondence to: Pest Management Science Lukasz L. Stelinski, Entomology and Nematology Department, Citrus Research & Education Center, University of Florida, 700 Experiment Station Road, Lake Alfred, FL 33850. Ph: 863 956 8851. Fax: 863 956 4631. Email: [email protected] Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions Siddharth Tiwari and Lukasz L. Stelinski* Entomology and Nematology Department, Citrus Research and Education Center, University of Florida, Lake Alfred, FL 33850 *Corresponding author Accepted Article This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ps.3468. © 2012 Society of Chemical Industry Abstract BACKGROUND:The Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), is the most destructive pest of citrus in Florida. The development of insecticide resistance in several populations of D. citrihas been documented. There is an urgent need to develop and integrate novel tools for the successful management of D. citri and also to prevent the development of insecticide resistance. RESULTS:We investigated the effects of a relatively newer chemistry, cyantraniliprole, against D. citri. The contact toxicity of cyantraniliprole was 297 fold higher against D. citrithan its primary parasitoid,Tamarixia radiata(Hymenoptera: Eulophidae). D. citrisettled and fed less on cyantraniliprole-treated plants than controls at concentrations as low as 0.025 and 0.125 µg AI mL-1, respectively. D. citri egg production, first instar emergence and adult emergence were significantly reduced on plants treated with 0.25, 0.02 and 0.25 µg AI mL-1of cyantraniliprole, respectively, when compared with control plants. -

Estimated Impact of Neonicotinoid Insecticides on Pest Management Practices and Costs for U.S
The Value of Neonicotinoids in North American Agriculture: AgInfomatics Estimated Impact of Neonicotinoid Insecticides on Pest Management Practices and Costs for U.S. Corn, Soybean, Wheat, Cotton and Sorghum Farmers 2014 This report series, researched and produced by AgInfomatics, LLC, is a comprehensive analysis of the economic and societal benefits of nitroguanidine neonicotinoid insecticides in North America. The research was sponsored by Bayer CropScience, Syngenta and Valent in support of regulatory review processes in the United States and Canada, with Mitsui providing additional support for the turf and ornamental studies. AgInfomatics, an agricultural consulting firm established in 1995 by professors from the University of Wisconsin-Madison and Washington State University, conducted independent analyses exploring the answer to the question: What would happen if neonicotinoids were no longer available? Comparing that answer to current product use revealed the value of neonicotinoids. Robust quantitative and qualitative study methods included econometrics modeling of insecticide use, crop yield data and market impacts; surveys of growers, professional applicators and consumers; regional listening panel sessions; and in-depth case studies. Active ingredients in the study included clothianidin, dinotefuran, imidacloprid and thiamethoxam. The Value of Neonicotinoids in North American The Value of Neonicotinoids in Turf and Agriculture Ornamentals Reports include: Reports include: Estimated Impact of Neonicotinoid Insecticides on Estimating the Economic Value of Neonicotinoid Pest Management Practices and Costs for U.S. Corn, Insecticides on Flowers, Shrubs, Home Lawns and Soybean, Wheat, Cotton and Sorghum Farmers Trees in the Homescape Methods and Assumptions for Estimating the The Value of Neonicotinoids to Turf and Ornamental Impact of Neonicotinoid Insecticides on Pest Professionals Management Practices and Costs for U.S. -

The Value of Neonicotinoids in Turf and Ornamentals: Aginfomatics
The Value of Neonicotinoids in Turf and Ornamentals: AgInfomatics A Case Study of Neonicotinoid Use for Controlling Chinch Bug in Florida St. Augustinegrass 2014 This report series, researched and produced by AgInfomatics, LLC, is a comprehensive analysis of the economic and societal benefits of nitroguanidine neonicotinoid insecticides in North America. The research was sponsored by Bayer CropScience, Syngenta and Valent in support of regulatory review processes in the United States and Canada, with Mitsui providing additional support for the turf and ornamental studies. AgInfomatics, an agricultural consulting firm established in 1995 by professors from the University of Wisconsin-Madison and Washington State University, conducted independent analyses exploring the answer to the question: What would happen if neonicotinoids were no longer available? Comparing that answer to current product use revealed the value of neonicotinoids. Robust quantitative and qualitative study methods included econometrics modeling of insecticide use, crop yield data and market impacts; surveys of growers, professional applicators and consumers; regional listening panel sessions; and in-depth case studies. Active ingredients in the study included clothianidin, dinotefuran, imidacloprid and thiamethoxam. The Value of Neonicotinoids in North American The Value of Neonicotinoids in Turf and Agriculture Ornamentals Reports include: Reports include: Estimated Impact of Neonicotinoid Insecticides on Estimating the Economic Value of Neonicotinoid Pest Management Practices and Costs for U.S. Corn, Insecticides on Flowers, Shrubs, Home Lawns and Soybean, Wheat, Cotton and Sorghum Farmers Trees in the Homescape Methods and Assumptions for Estimating the The Value of Neonicotinoids to Turf and Ornamental Impact of Neonicotinoid Insecticides on Pest Professionals Management Practices and Costs for U.S.